| << Chapter < Page | Chapter >> Page > |
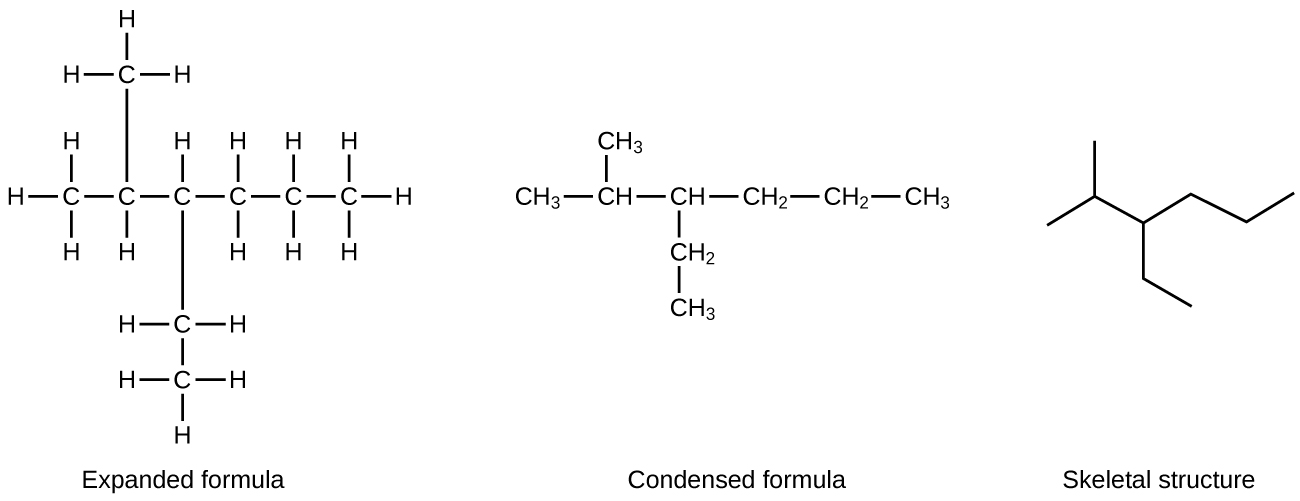
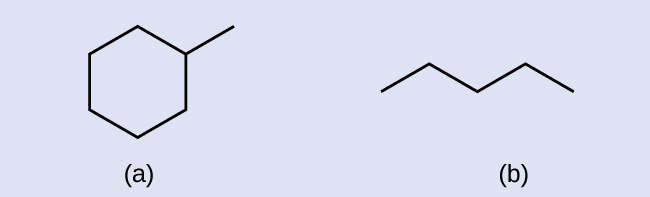
A common method used by organic chemists to simplify the drawings of larger molecules is to use a skeletal structure (also called a line-angle structure). In this type of structure, carbon atoms are not symbolized with a C, but represented by each end of a line or bend in a line. Hydrogen atoms are not drawn if they are attached to a carbon. Other atoms besides carbon and hydrogen are represented by their elemental symbols. [link] shows three different ways to draw the same structure.
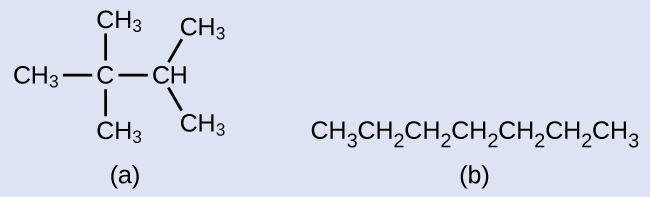

Location of the hydrogen atoms:
C 9 H 20
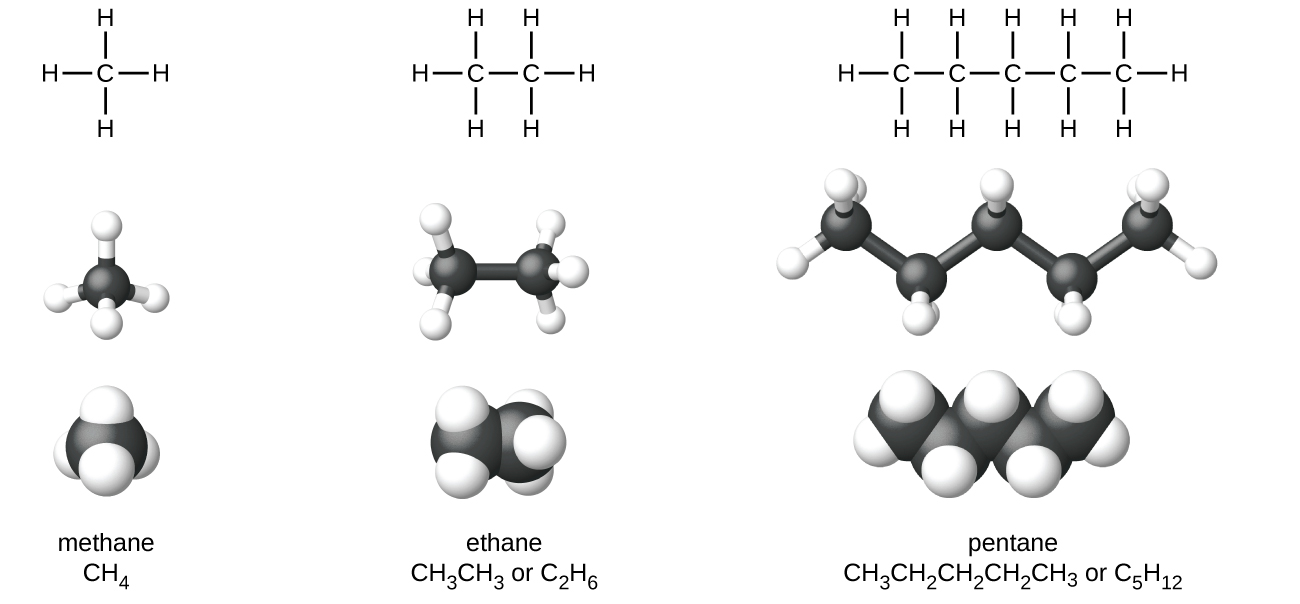
All alkanes are composed of carbon and hydrogen atoms, and have similar bonds, structures, and formulas; noncyclic alkanes all have a formula of C n H 2n+2 . The number of carbon atoms present in an alkane has no limit. Greater numbers of atoms in the molecules will lead to stronger intermolecular attractions (dispersion forces) and correspondingly different physical properties of the molecules. Properties such as melting point and boiling point ( [link] ) usually change smoothly and predictably as the number of carbon and hydrogen atoms in the molecules change.
| Properties of Some Alkanes Physical properties for C 4 H 10 and heavier molecules are those of the normal isomer , n -butane, n -pentane, etc. | |||||
|---|---|---|---|---|---|
| Alkane | Molecular Formula | Melting Point (°C) | Boiling Point (°C) | Phase at STP STP indicates a temperature of 0 °C and a pressure of 1 atm. | Number of Structural Isomers |
| methane | CH 4 | –182.5 | –161.5 | gas | 1 |
| ethane | C 2 H 6 | –183.3 | –88.6 | gas | 1 |
| propane | C 3 H 8 | –187.7 | –42.1 | gas | 1 |
| butane | C 4 H 10 | –138.3 | –0.5 | gas | 2 |
| pentane | C 5 H 12 | –129.7 | 36.1 | liquid | 3 |
| hexane | C 6 H 14 | –95.3 | 68.7 | liquid | 5 |
| heptane | C 7 H 16 | –90.6 | 98.4 | liquid | 9 |
| octane | C 8 H 18 | –56.8 | 125.7 | liquid | 18 |
| nonane | C 9 H 20 | –53.6 | 150.8 | liquid | 35 |
| decane | C 10 H 22 | –29.7 | 174.0 | liquid | 75 |
| tetradecane | C 14 H 30 | 5.9 | 253.5 | solid | 1858 |
| octadecane | C 18 H 38 | 28.2 | 316.1 | solid | 60,523 |
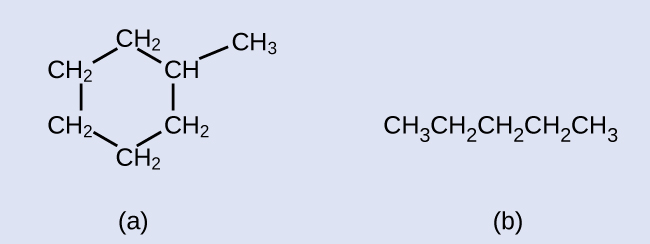
Hydrocarbons with the same formula, including alkanes, can have different structures. For example, two alkanes have the formula C 4 H 10 : They are called n -butane and 2-methylpropane (or isobutane), and have the following Lewis structures:

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?