| << Chapter < Page | Chapter >> Page > |
Se 2− , the selenide ion
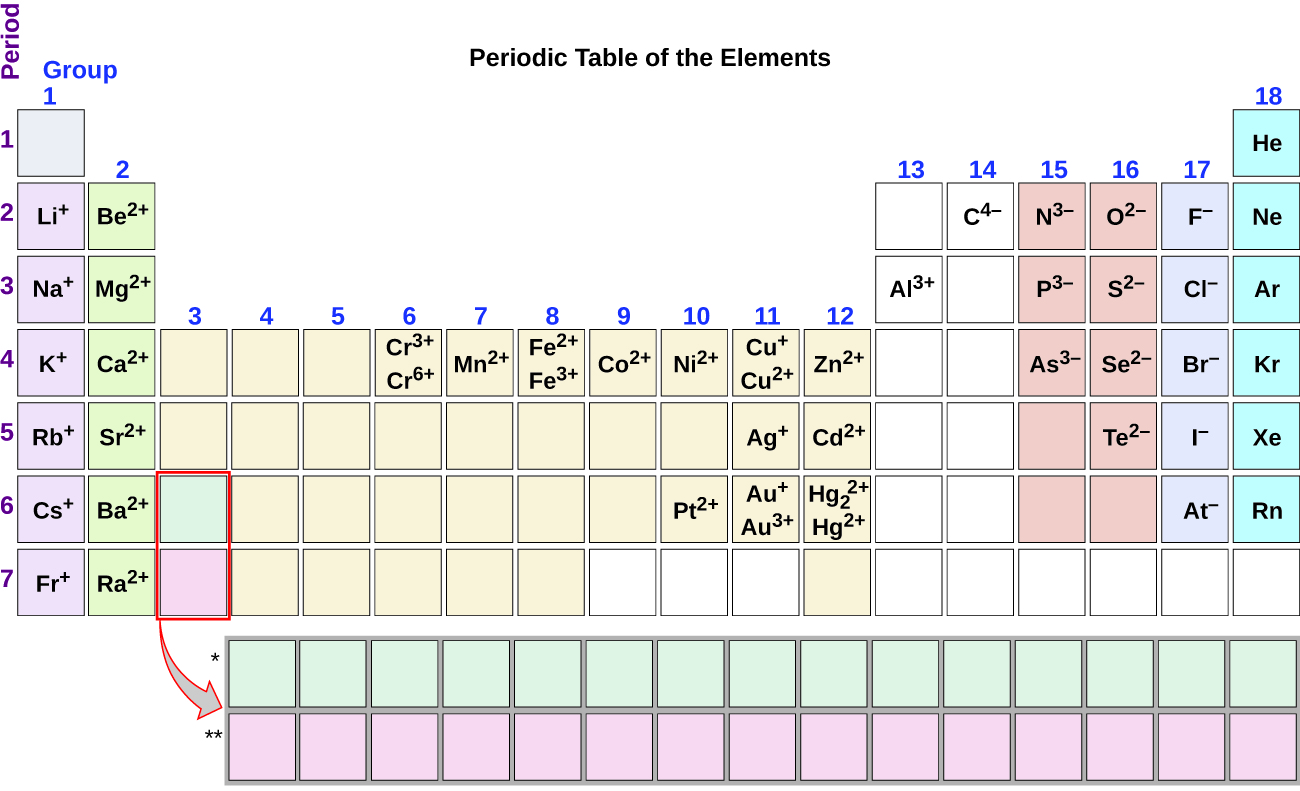
Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3−. The symbol for the ion is N 3− , and it is called a nitride ion.
Al will form a cation with a charge of 3+: Al 3+ , an aluminum ion. Carbon will form an anion with a charge of 4−: C 4− , a carbide ion.
The ions that we have discussed so far are called monatomic ions , that is, they are ions formed from only one atom. We also find many polyatomic ions . These ions, which act as discrete units, are electrically charged molecules (a group of bonded atoms with an overall charge). Some of the more important polyatomic ions are listed in [link] . Oxyanions are polyatomic ions that contain one or more oxygen atoms. At this point in your study of chemistry, you should memorize the names, formulas, and charges of the most common polyatomic ions. Because you will use them repeatedly, they will soon become familiar.
| Common Polyatomic Ions | |||
|---|---|---|---|
| Name | Formula | Related Acid | Formula |
| ammonium | |||
| hydronium | |||
| oxide | |||
| peroxide | |||
| hydroxide | |||
| acetate | acetic acid | CH 3 COOH | |
| cyanide | CN − | hydrocyanic acid | HCN |
| azide | hydrazoic acid | HN 3 | |
| carbonate | carbonic acid | H 2 CO 3 | |
| bicarbonate | |||
| nitrate | nitric acid | HNO 3 | |
| nitrite | nitrous acid | HNO 2 | |
| sulfate | sulfiric acid | H 2 SO 4 | |
| hydrogen sulfate | |||
| sulfite | sulfurous acid | H 2 SO 3 | |
| hydrogen sulfite | |||
| phosphate | phosphoric acid | H 3 PO 4 | |
| hydrogen phosphate | |||
| dihydrogen phosphate | |||
| perchlorate | perchloric acid | HClO 4 | |
| chlorate | chloric acid | HClO 3 | |
| chlorite | chlorous acid | HClO 2 | |
| hypochlorite | ClO − | hypochlorous acid | HClO |
| chromate | chromic acid | H 2 Cr 2 O 4 | |
| dichromate | dichromic acid | H 2 Cr 2 O 7 | |
| permanganate | permanganic acid | HMnO 4 |

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?