| << Chapter < Page | Chapter >> Page > |
Ionic oxides all contain the oxide ion, a very powerful hydrogen ion acceptor. With the exception of the very insoluble aluminum oxide, Al 2 O 3 , tin(IV), SnO 2 , and lead(IV), PbO 2 , the oxides of the representative metals react with acids to form salts. Some equations for these reactions are:
The oxides of the metals of groups 1 and 2 and of thallium(I) oxide react with water and form hydroxides. Examples of such reactions are:
The oxides of the alkali metals have little industrial utility, unlike magnesium oxide, calcium oxide, and aluminum oxide. Magnesium oxide is important in making firebrick, crucibles, furnace linings, and thermal insulation—applications that require chemical and thermal stability. Calcium oxide, sometimes called quicklime or lime in the industrial market, is very reactive, and its principal uses reflect its reactivity. Pure calcium oxide emits an intense white light when heated to a high temperature (as illustrated in [link] ). Blocks of calcium oxide heated by gas flames were the stage lights in theaters before electricity was available. This is the source of the phrase “in the limelight.”
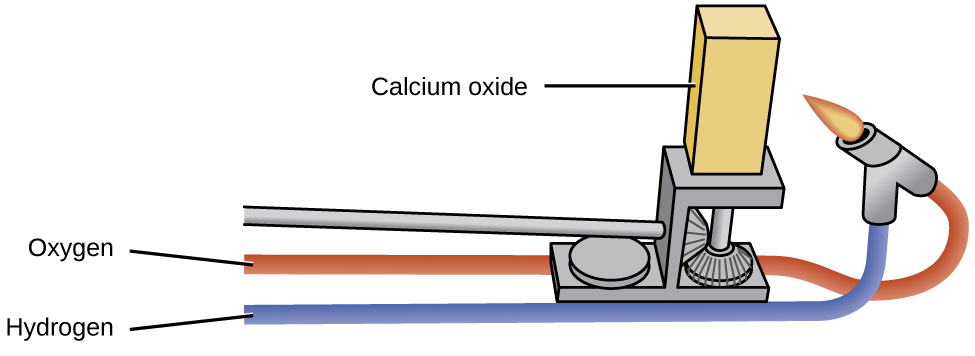
Calcium oxide and calcium hydroxide are inexpensive bases used extensively in chemical processing, although most of the useful products prepared from them do not contain calcium. Calcium oxide, CaO, is made by heating calcium carbonate, CaCO 3 , which is widely and inexpensively available as limestone or oyster shells:
Although this decomposition reaction is reversible, it is possible to obtain a 100% yield of CaO by allowing the CO 2 to escape. It is possible to prepare calcium hydroxide by the familiar acid-base reaction of a soluble metal oxide with water:
Both CaO and Ca(OH) 2 are useful as bases; they accept protons and neutralize acids.
Alumina (Al 2 O 3 ) occurs in nature as the mineral corundum, a very hard substance used as an abrasive for grinding and polishing. Corundum is important to the jewelry trade as ruby and sapphire. The color of ruby is due to the presence of a small amount of chromium; other impurities produce the wide variety of colors possible for sapphires. Artificial rubies and sapphires are now manufactured by melting aluminum oxide (melting point = 2050 °C) with small amounts of oxides to produce the desired colors and cooling the melt in such a way as to produce large crystals. Ruby lasers use synthetic ruby crystals.
Zinc oxide, ZnO, was a useful white paint pigment; however, pollutants tend to discolor the compound. The compound is also important in the manufacture of automobile tires and other rubber goods, and in the preparation of medicinal ointments. For example, zinc-oxide-based sunscreens, as shown in [link] , help prevent sunburn. The zinc oxide in these sunscreens is present in the form of very small grains known as nanoparticles. Lead dioxide is a constituent of charged lead storage batteries. Lead(IV) tends to revert to the more stable lead(II) ion by gaining two electrons, so lead dioxide is a powerful oxidizing agent.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?