| << Chapter < Page | Chapter >> Page > |
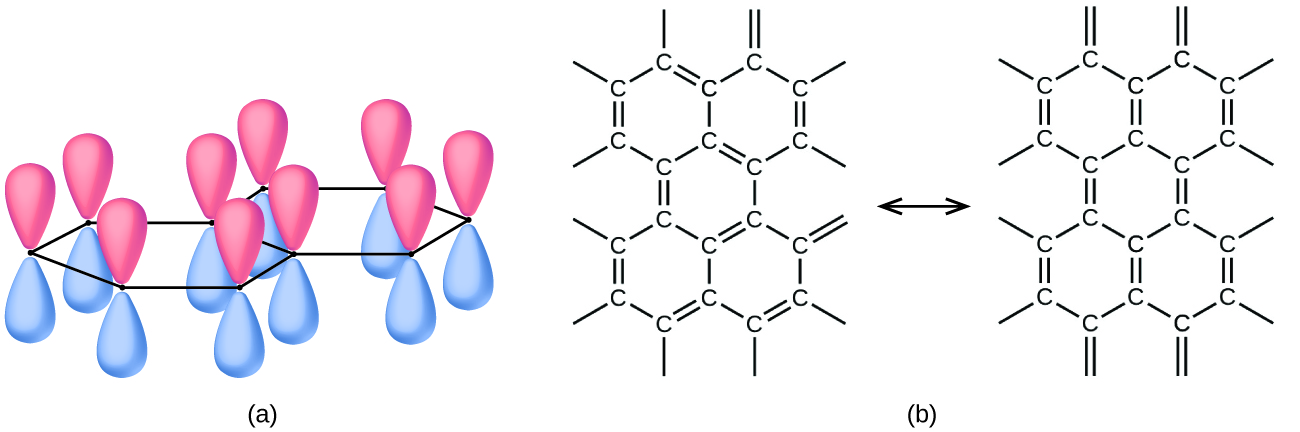
Atoms within a graphite layer are bonded together tightly by the σ and π bonds; however, the forces between layers are weak. London dispersion forces hold the layers together. To learn more, see the discussion of these weak forces in the chapter on liquids and solids. The weak forces between layers give graphite the soft, flaky character that makes it useful as the so-called “lead” in pencils and the slippery character that makes it useful as a lubricant. The loosely held electrons in the resonating π bonds can move throughout the solid and are responsible for the electrical conductivity of graphite.
Other forms of elemental carbon include carbon black, charcoal, and coke. Carbon black is an amorphous form of carbon prepared by the incomplete combustion of natural gas, CH 4 . It is possible to produce charcoal and coke by heating wood and coal, respectively, at high temperatures in the absence of air.
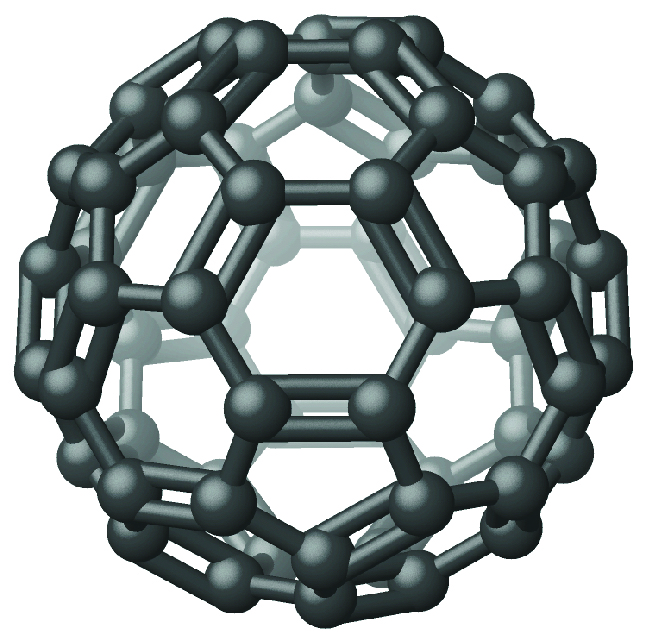
Recently, new forms of elemental carbon molecules have been identified in the soot generated by a smoky flame and in the vapor produced when graphite is heated to very high temperatures in a vacuum or in helium. One of these new forms, first isolated by Professor Richard Smalley and coworkers at Rice University, consists of icosahedral (soccer-ball-shaped) molecules that contain 60 carbon atoms, C 60 . This is buckminsterfullerene (often called bucky balls) after the architect Buckminster Fuller , who designed domed structures, which have a similar appearance ( [link] ).
Graphene and carbon nanotubes are two recently discovered allotropes of carbon. Both of the forms bear some relationship to graphite. Graphene is a single layer of graphite (one atom thick), as illustrated in [link] , whereas carbon nanotubes roll the layer into a small tube, as illustrated in [link] .
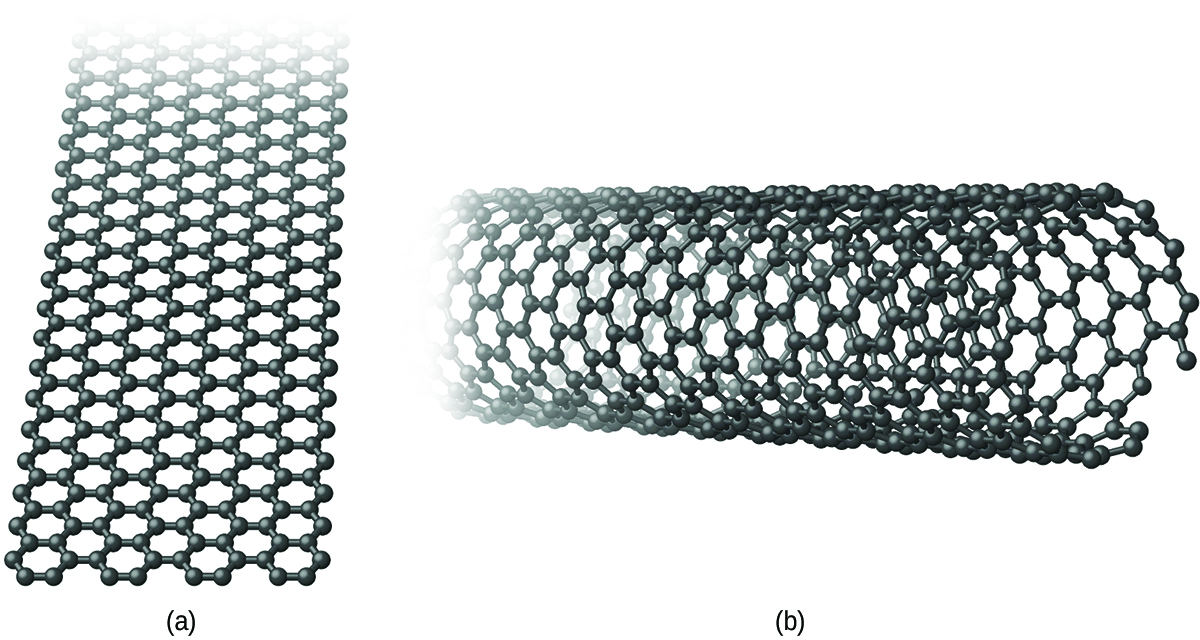
Graphene is a very strong, lightweight, and efficient conductor of heat and electricity discovered in 2003. As in graphite, the carbon atoms form a layer of six-membered rings with sp 2 -hybridized carbon atoms at the corners. Resonance stabilizes the system and leads to its conductivity. Unlike graphite, there is no stacking of the layers to give a three-dimensional structure. Andre Geim and Kostya Novoselov at the University of Manchester won the 2010 Nobel Prize in Physics for their pioneering work characterizing graphene.
The simplest procedure for preparing graphene is to use a piece of adhesive tape to remove a single layer of graphene from the surface of a piece of graphite. This method works because there are only weak London dispersion forces between the layers in graphite. Alternative methods are to deposit a single layer of carbon atoms on the surface of some other material (ruthenium, iridium, or copper) or to synthesize it at the surface of silicon carbide via the sublimation of silicon.
There currently are no commercial applications of graphene. However, its unusual properties, such as high electron mobility and thermal conductivity, should make it suitable for the manufacture of many advanced electronic devices and for thermal management applications.
Carbon nanotubes are carbon allotropes, which have a cylindrical structure. Like graphite and graphene, nanotubes consist of rings of sp 2 -hybridized carbon atoms. Unlike graphite and graphene, which occur in layers, the layers wrap into a tube and bond together to produce a stable structure. The walls of the tube may be one atom or multiple atoms thick.
Carbon nanotubes are extremely strong materials that are harder than diamond. Depending upon the shape of the nanotube, it may be a conductor or semiconductor. For some applications, the conducting form is preferable, whereas other applications utilize the semiconducting form.
The basis for the synthesis of carbon nanotubes is the generation of carbon atoms in a vacuum. It is possible to produce carbon atoms by an electrical discharge through graphite, vaporization of graphite with a laser, and the decomposition of a carbon compound.
The strength of carbon nanotubes will eventually lead to some of their most exciting applications, as a thread produced from several nanotubes will support enormous weight. However, the current applications only employ bulk nanotubes. The addition of nanotubes to polymers improves the mechanical, thermal, and electrical properties of the bulk material. There are currently nanotubes in some bicycle parts, skis, baseball bats, fishing rods, and surfboards.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?