| << Chapter < Page | Chapter >> Page > |
There are times when one equilibrium reaction does not adequately describe the system being studied. Sometimes we have more than one type of equilibrium occurring at once (for example, an acid-base reaction and a precipitation reaction).
The ocean is a unique example of a system with multiple equilibria , or multiple states of solubility equilibria working simultaneously. Carbon dioxide in the air dissolves in sea water, forming carbonic acid (H 2 CO 3 ). The carbonic acid then ionizes to form hydrogen ions and bicarbonate ions which can further ionize into more hydrogen ions and carbonate ions
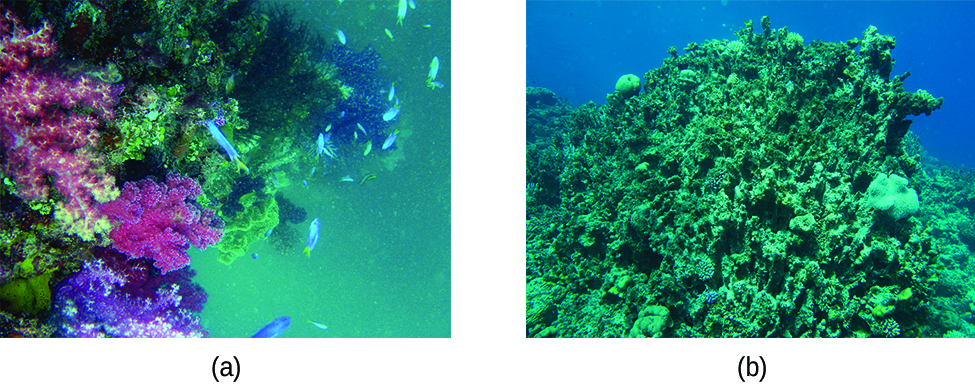
The excess H + ions make seawater more acidic. Increased ocean acidification can then have negative impacts on reef-building coral, as they cannot absorb the calcium carbonate they need to grow and maintain their skeletons ( [link] ). This in turn disrupts the local biosystem that depends upon the health of the reefs for its survival. If enough local reefs are similarly affected, the disruptions to sea life can be felt globally. The world’s oceans are presently in the midst of a period of intense acidification, believed to have begun in the mid-nineteenth century, and which is now accelerating at a rate faster than any change to oceanic pH in the last 20 million years.
Learn more about ocean acidification and how it affects other marine creatures.
This site has detailed information about how ocean acidification specifically affects coral reefs.
Slightly soluble solids derived from weak acids generally dissolve in strong acids, unless their solubility products are extremely small. For example, we can dissolve CuCO 3 , FeS, and Ca 3 (PO 4 ) 2 in HCl because their basic anions react to form weak acids (H 2 CO 3 , H 2 S, and The resulting decrease in the concentration of the anion results in a shift of the equilibrium concentrations to the right in accordance with Le Châtelier’s principle.
Of particular relevance to us is the dissolution of hydroxylapatite, Ca 5 (PO 4 ) 3 OH, in acid. Apatites are a class of calcium phosphate minerals ( [link] ); a biological form of hydroxylapatite is found as the principal mineral in the enamel of our teeth. A mixture of hydroxylapatite and water (or saliva) contains an equilibrium mixture of solid Ca 5 (PO 4 ) 3 OH and dissolved Ca 2+ , and OH – ions:

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?