| << Chapter < Page | Chapter >> Page > |
Acetic acid, CH 3 CO 2 H, is a weak acid. When we add acetic acid to water, it ionizes to a small extent according to the equation:
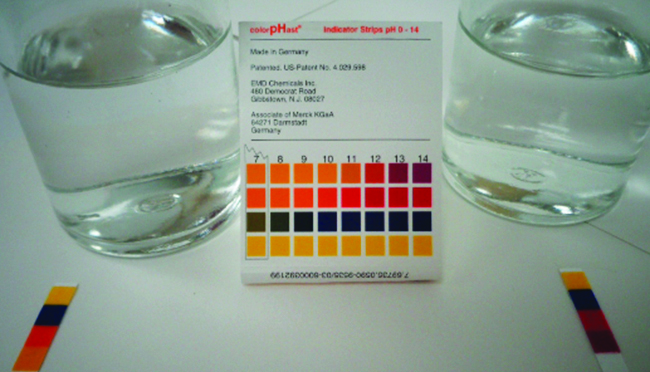
giving an equilibrium mixture with most of the acid present in the nonionized (molecular) form. This equilibrium, like other equilibria, is dynamic; acetic acid molecules donate hydrogen ions to water molecules and form hydronium ions and acetate ions at the same rate that hydronium ions donate hydrogen ions to acetate ions to reform acetic acid molecules and water molecules. We can tell by measuring the pH of an aqueous solution of known concentration that only a fraction of the weak acid is ionized at any moment ( [link] ). The remaining weak acid is present in the nonionized form.
For acetic acid, at equilibrium:

| Ionization Constants of Some Weak Acids | |
|---|---|
| Ionization Reaction | K a at 25 °C |
| 1.2 10 −2 | |
| 3.5 10 −4 | |
| 4.6 10 −4 | |
| 2 10 −4 | |
| 1.8 10 −4 | |
| 1.8 10 −5 | |
| 2.9 10 −8 | |
| 2.8 10 −9 | |
| 4.9 10 −10 |
[link] gives the ionization constants for several weak acids; additional ionization constants can be found in Appendix H .
At equilibrium, a solution of a weak base in water is a mixture of the nonionized base, the conjugate acid of the weak base, and hydroxide ion with the nonionized base present in the greatest concentration. Thus, a weak base increases the hydroxide ion concentration in an aqueous solution (but not as much as the same amount of a strong base).
For example, a solution of the weak base trimethylamine, (CH 3 ) 3 N, in water reacts according to the equation:
giving an equilibrium mixture with most of the base present as the nonionized amine. This equilibrium is analogous to that described for weak acids.
We can confirm by measuring the pH of an aqueous solution of a weak base of known concentration that only a fraction of the base reacts with water ( [link] ). The remaining weak base is present as the unreacted form. The equilibrium constant for the ionization of a weak base, K b , is called the ionization constant of the weak base, and is equal to the reaction quotient when the reaction is at equilibrium. For trimethylamine, at equilibrium:

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?