| << Chapter < Page | Chapter >> Page > |
Recall that a small K c means that very little of the reactants form products and a large K c means that most of the reactants form products. If the system can be arranged so it starts “close” to equilibrium, then if the change ( x ) is small compared to any initial concentrations, it can be neglected. Small is usually defined as resulting in an error of less than 5%. The following two examples demonstrate this.
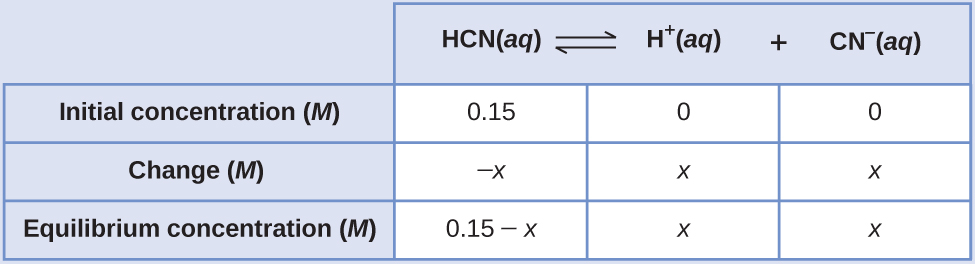
The exact solution may be obtained using the quadratic formula with
solving
Thus [H + ] = [CN – ] = x = 8.6 10 –6 M and [HCN] = 0.15 – x = 0.15 M .
In this case, chemical intuition can provide a simpler solution. From the equilibrium constant and the initial conditions, x must be small compared to 0.15 M . More formally, if then 0.15 – x ≈ 0.15. If this assumption is true, then it simplifies obtaining x
In this example, solving the exact (quadratic) equation and using approximations gave the same result to two significant figures. While most of the time the approximation is a bit different from the exact solution, as long as the error is less than 5%, the approximate solution is considered valid. In this problem, the 5% applies to IF (0.15 – x ) ≈ 0.15 M , so if
is less than 5%, as it is in this case, the assumption is valid. The approximate solution is thus a valid solution.
Assume that x is much less than 0.25 M and calculate the error in your assumption.
[NH 3 ] = 0.25 M , error = 0.84%
The second example requires that the original information be processed a bit, but it still can be solved using a small x approximation.
If 0.010 mol Cu 2+ is added to 1.00 L of a solution that is 1.00 M NH 3 what are the concentrations when the system comes to equilibrium?
Using these “shifted” values as initial concentrations with x as the free copper(II) ion concentration at equilibrium gives this ICE table.
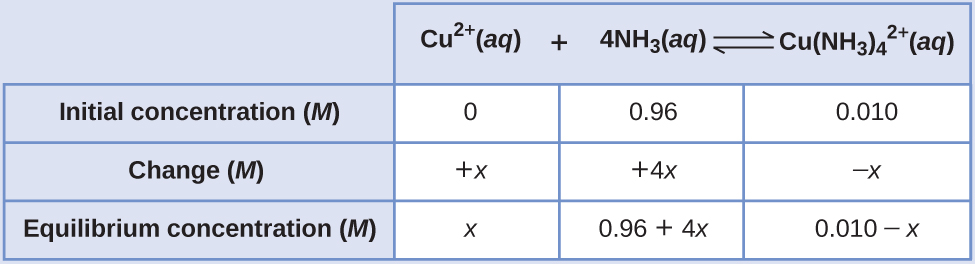
Since we are starting close to equilibrium, x should be small so that
Select the smallest concentration for the 5% rule.
This is much less than 5%, so the assumptions are valid. The concentrations at equilibrium are
By starting with the maximum amount of product, this system was near equilibrium and the change ( x ) was very small. With only a small change required to get to equilibrium, the equation for x was greatly simplified and gave a valid result well within the 5% error maximum.
With such a large equilibrium constant, first form as much product as possible, then assume that only a small amount ( x ) of the product shifts left. Calculate the error in your assumption.
[NH 3 ] = 0.50 M , [Ni 2+ ] = 2.9 10 –8 M , error = 1.2 10 –5 %

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?