| << Chapter < Page | Chapter >> Page > |
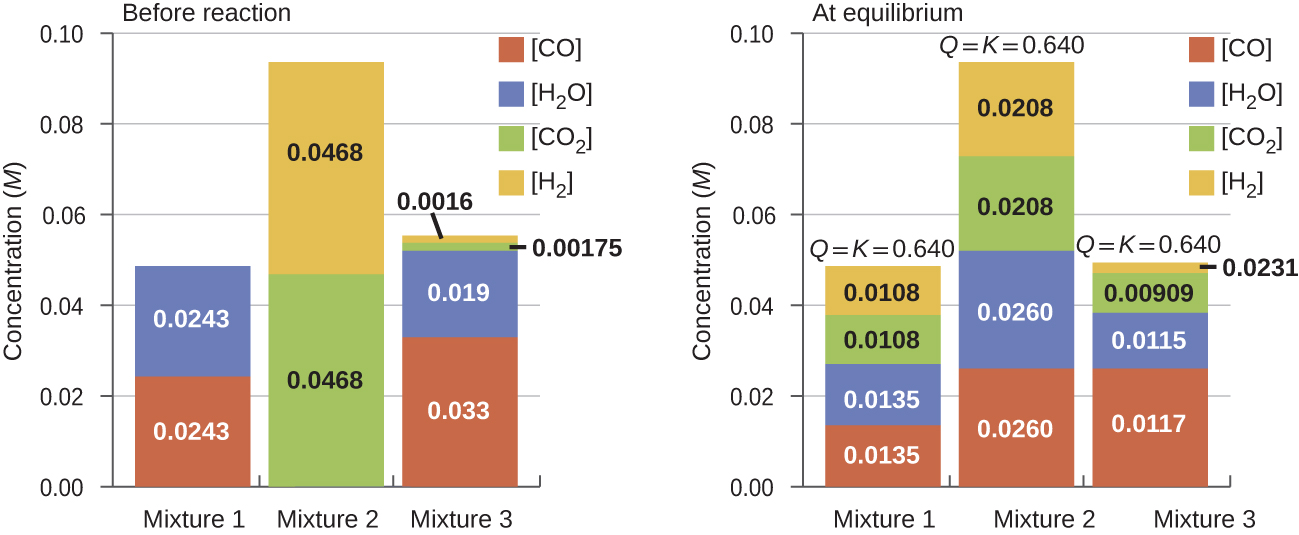
Determine in which direction the reaction proceeds as it goes to equilibrium in each of the three experiments shown.
| Reactants/Products | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| [CO] i | 0.0203 M | 0.011 M | 0.0094 M |
| [H 2 O] i | 0.0203 M | 0.0011 M | 0.0025 M |
| [CO 2 ] i | 0.0040 M | 0.037 M | 0.0015 M |
| [H 2 ] i | 0.0040 M | 0.046 M | 0.0076 M |
Q c < K c (0.039<0.64)
The reaction will shift to the right.
Experiment 2:
Q c > K c (140>0.64)
The reaction will shift to the left.
Experiment 3:
Q c < K c (0.48<0.64)
The reaction will shift to the right.
(a) A 1.00-L flask containing 0.0500 mol of NO(g), 0.0155 mol of Cl2(g), and 0.500 mol of NOCl:
(b) A 5.0-L flask containing 17 g of NH 3 , 14 g of N 2 , and 12 g of H 2 :
(c) A 2.00-L flask containing 230 g of SO 3 (g):
(a) Q c = 6.45 10 3 , shifts right. (b) Q c = 0.12, shifts left. (c) Q c = 0, shifts right
In [link] , it was mentioned that the common practice is to omit units when evaluating reaction quotients and equilibrium constants. It should be pointed out that using concentrations in these computations is a convenient but simplified approach that sometimes leads to results that seemingly conflict with the law of mass action. For example, equilibria involving aqueous ions often exhibit equilibrium constants that vary quite significantly (are not constant) at high solution concentrations. This may be avoided by computing K c values using the activities of the reactants and products in the equilibrium system instead of their concentrations. The activity of a substance is a measure of its effective concentration under specified conditions. While a detailed discussion of this important quantity is beyond the scope of an introductory text, it is necessary to be aware of a few important aspects:
As a consequence of this last consideration, Q c and K c expressions do not contain terms for solids or liquids (being numerically equal to 1, these terms have no effect on the expression's value) . Several examples of equilibria yielding such expressions will be encountered in this section.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?