| << Chapter < Page | Chapter >> Page > |
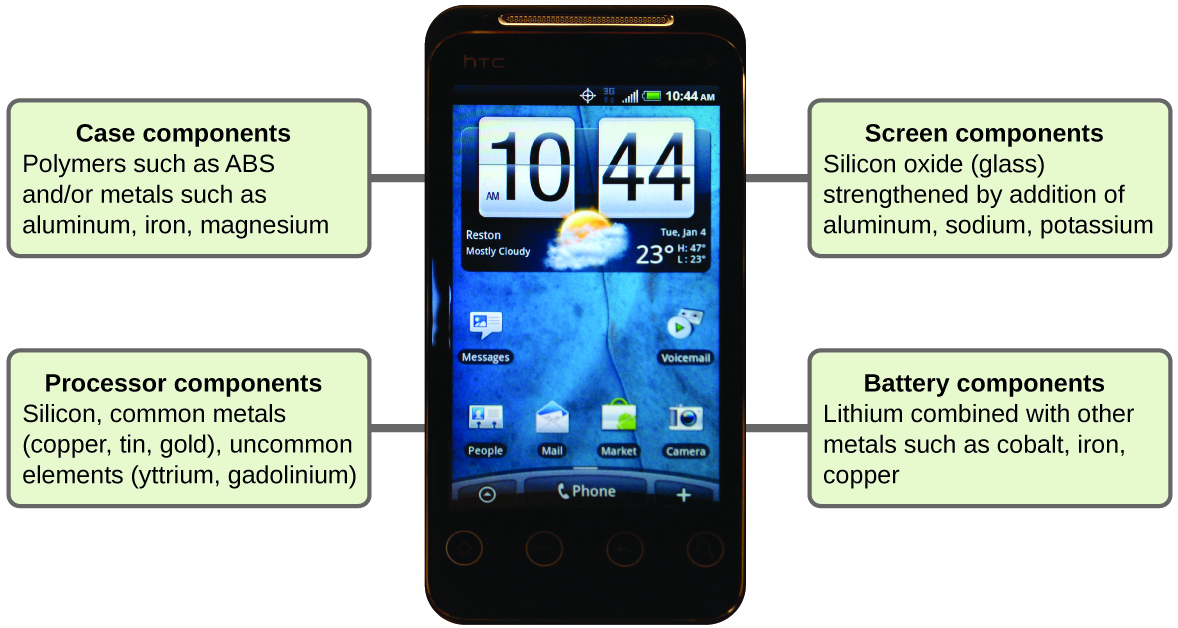
Imagine how different your life would be without cell phones ( [link] ) and other smart devices. Cell phones are made from numerous chemical substances, which are extracted, refined, purified, and assembled using an extensive and in-depth understanding of chemical principles. About 30% of the elements that are found in nature are found within a typical smart phone. The case/body/frame consists of a combination of sturdy, durable polymers comprised primarily of carbon, hydrogen, oxygen, and nitrogen [acrylonitrile butadiene styrene (ABS) and polycarbonate thermoplastics], and light, strong, structural metals, such as aluminum, magnesium, and iron. The display screen is made from a specially toughened glass (silica glass strengthened by the addition of aluminum, sodium, and potassium) and coated with a material to make it conductive (such as indium tin oxide). The circuit board uses a semiconductor material (usually silicon); commonly used metals like copper, tin, silver, and gold; and more unfamiliar elements such as yttrium, praseodymium, and gadolinium. The battery relies upon lithium ions and a variety of other materials, including iron, cobalt, copper, polyethylene oxide, and polyacrylonitrile.
Matter is anything that occupies space and has mass. The basic building block of matter is the atom, the smallest unit of an element that can enter into combinations with atoms of the same or other elements. In many substances, atoms are combined into molecules. On earth, matter commonly exists in three states: solids, of fixed shape and volume; liquids, of variable shape but fixed volume; and gases, of variable shape and volume. Under high-temperature conditions, matter also can exist as a plasma. Most matter is a mixture: It is composed of two or more types of matter that can be present in varying amounts and can be separated by physical means. Heterogeneous mixtures vary in composition from point to point; homogeneous mixtures have the same composition from point to point. Pure substances consist of only one type of matter. A pure substance can be an element, which consists of only one type of atom and cannot be broken down by a chemical change, or a compound, which consists of two or more types of atoms.
Why do we use an object's mass, rather than its weight, to indicate the amount of matter it contains?
What properties distinguish solids from liquids? Liquids from gases? Solids from gases?
Liquids can change their shape (flow); solids can’t. Gases can undergo large volume changes as pressure changes; liquids do not. Gases flow and change volume; solids do not.
How does a heterogeneous mixture differ from a homogeneous mixture? How are they similar?
How does a homogeneous mixture differ from a pure substance? How are they similar?
The mixture can have a variety of compositions; a pure substance has a definite composition. Both have the same composition from point to point.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?