-
Home
- Chemistry
- Chemistry
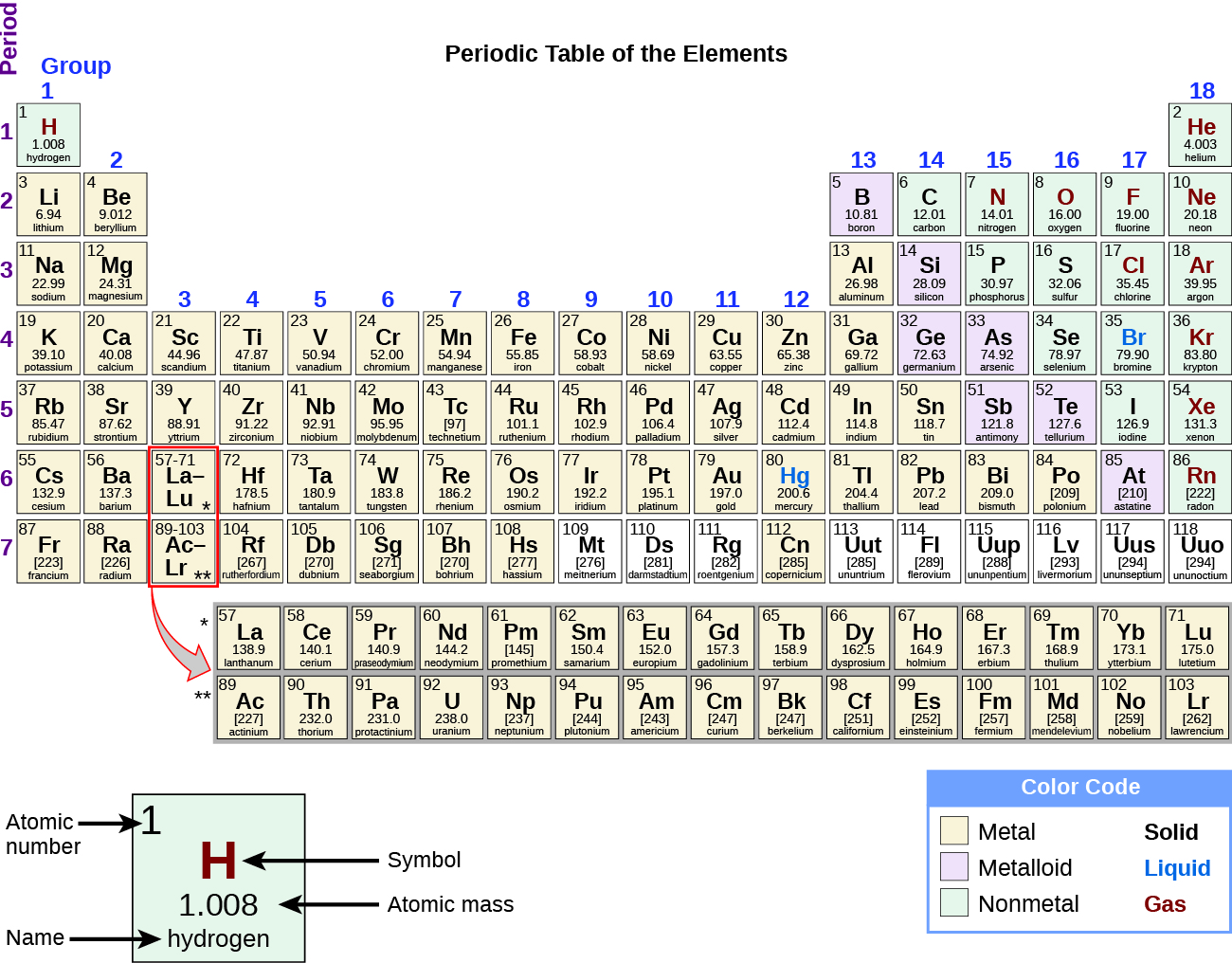
- The periodic table
Questions & Answers
Discuss the differences between taste and flavor, including how other sensory inputs contribute to our perception of flavor.
taste refers to your understanding of the flavor . while flavor one The other hand is refers to sort of just a blend things.
Faith
While taste primarily relies on our taste buds, flavor involves a complex interplay between taste and aroma
Kamara
which drugs can we use for ulcers
Omeprazole
Cimetidine / Tagament
For the complicated once ulcer - kit
Patrick
what is the function of lymphatic system
to drain extracellular fluid all over the body.
asegid
The lymphatic system plays several crucial roles in the human body, functioning as a key component of the immune system and contributing to the maintenance of fluid balance. Its main functions include:
1. Immune Response: The lymphatic system produces and transports lymphocytes, which are a type of
asegid
to transport fluids fats proteins and lymphocytes to the blood stream as lymph
Adama
Anatomy is the identification and description of the structures of living things
Kamara
what's the difference between anatomy and physiology
Anatomy is the study of the structure of the body, while physiology is the study of the function of the body. Anatomy looks at the body's organs and systems, while physiology looks at how those organs and systems work together to keep the body functioning.
AI-Robot
what is enzymes all about?
Enzymes are proteins that help speed up chemical reactions in our bodies. Enzymes are essential for digestion, liver function and much more. Too much or too little of a certain enzyme can cause health problems
Kamara
how does the stomach protect itself from the damaging effects of HCl
little girl okay how does the stomach protect itself from the damaging effect of HCL
Wulku
it is because of the enzyme that the stomach produce that help the stomach from the damaging effect of HCL
Kamara
function of digestive system
function of digestive
Ali
what is the normal body temperature
please why 37 degree selcius normal temperature
Mark
the normal temperature is 37°c or 98.6 °Fahrenheit is important for maintaining the homeostasis in the body
the body regular this temperature through the process called thermoregulation which involves brain skin muscle and other organ working together to maintain stable internal temperature
Stephanie
anaemia is the decrease in RBC count hemoglobin count and PVC count
Eniola
what is the pH of the vagina
how does Lysin attack pathogens
Diya
I information on anatomy position and digestive system and there enzyme
anatomy of the female external genitalia
Organ Systems Of The Human Body (Continued)
Organ Systems Of The Human Body (Continued)
what's lochia albra
Kizito
Got questions? Join the online conversation and get instant answers!
Source:
OpenStax, Chemistry. OpenStax CNX. May 20, 2015 Download for free at http://legacy.cnx.org/content/col11760/1.9
Google Play and the Google Play logo are trademarks of Google Inc.