| << Chapter < Page | Chapter >> Page > |
In aerobic respiration, the final electron acceptor is an oxygen molecule, O 2 . If aerobic respiration occurs, then ATP will be produced using the energy of the high-energy electrons carried by NADH or FADH 2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD + for reuse as an electron carrier for glycolysis to continue. How is this done? Humans use an organic molecule (pyruvate/pyruvic acid)as the final electron acceptor. Processes that use an organic molecule to regenerate NAD + from NADH are collectively referred to as fermentation .
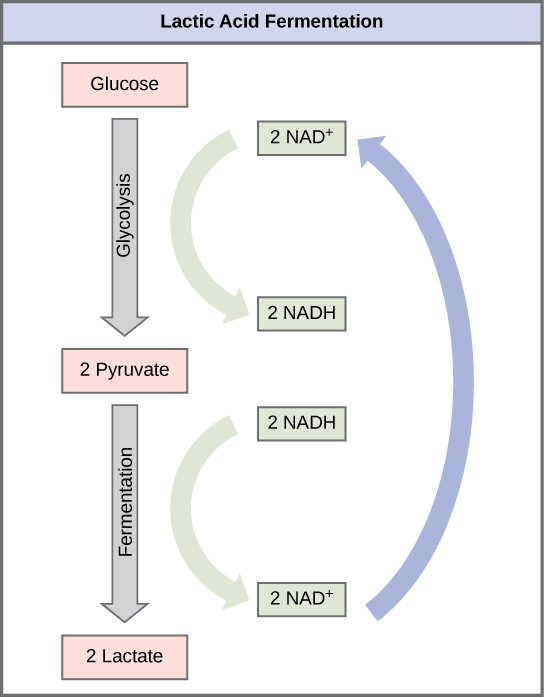
The fermentation method used by animals and some bacteria like those in yogurt is lactic acid fermentation ( [link] ). This occurs routinely in mammalian red blood cells and in skeletal muscle that has insufficient oxygen supply to allow aerobic respiration to continue (that is, in muscles used to the point of fatigue). In muscles, lactic acid produced by fermentation must be removed by the blood circulation and brought to the liver for further metabolism. The chemical reaction of lactic acid fermentation is the following:
Another familiar fermentation process is alcohol fermentation ( [link] ), which produces ethanol, an alcohol. The alcohol fermentation reaction is the following:
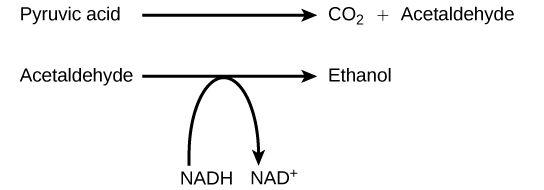
In the first reaction, a carboxyl group is removed from pyruvic acid, releasing carbon dioxide as a gas. The loss of carbon dioxide reduces the molecule by one carbon atom, making acetaldehyde. The second reaction removes an electron from NADH, forming NAD + and producing ethanol from the acetaldehyde, which accepts the electron. The fermentation of pyruvic acid by yeast produces the ethanol found in alcoholic beverages ( [link] ). If the carbon dioxide produced by the reaction is not vented from the fermentation chamber, for example in beer and sparkling wines, it remains dissolved in the medium until the pressure is released. Ethanol above 12 percent is toxic to yeast, so natural levels of alcohol in wine occur at a maximum of 12 percent.

If NADH cannot be metabolized through aerobic respiration, another electron acceptor is used. Most organisms will use some form of fermentation to accomplish the regeneration of NAD + , ensuring the continuation of glycolysis. The regeneration of NAD + in fermentation is not accompanied by ATP production; therefore, the potential for NADH to produce ATP using an electron transport chain is not utilized.

Notification Switch
Would you like to follow the 'Human biology' conversation and receive update notifications?