| << Chapter < Page | Chapter >> Page > |
Carbon is the fourth most abundant element in living organisms. Carbon is present in all organic molecules, and its role in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain energy, and many of these compounds from plants and algae have remained stored as fossilized carbon, which humans use as fuel. Since the 1800s, the use of fossil fuels has accelerated. As global demand for Earth’s limited fossil fuel supplies has risen since the beginning of the Industrial Revolution, the amount of carbon dioxide in our atmosphere has increased as the fuels are burned. This increase in carbon dioxide has been associated with climate change and is a major environmental concern worldwide.
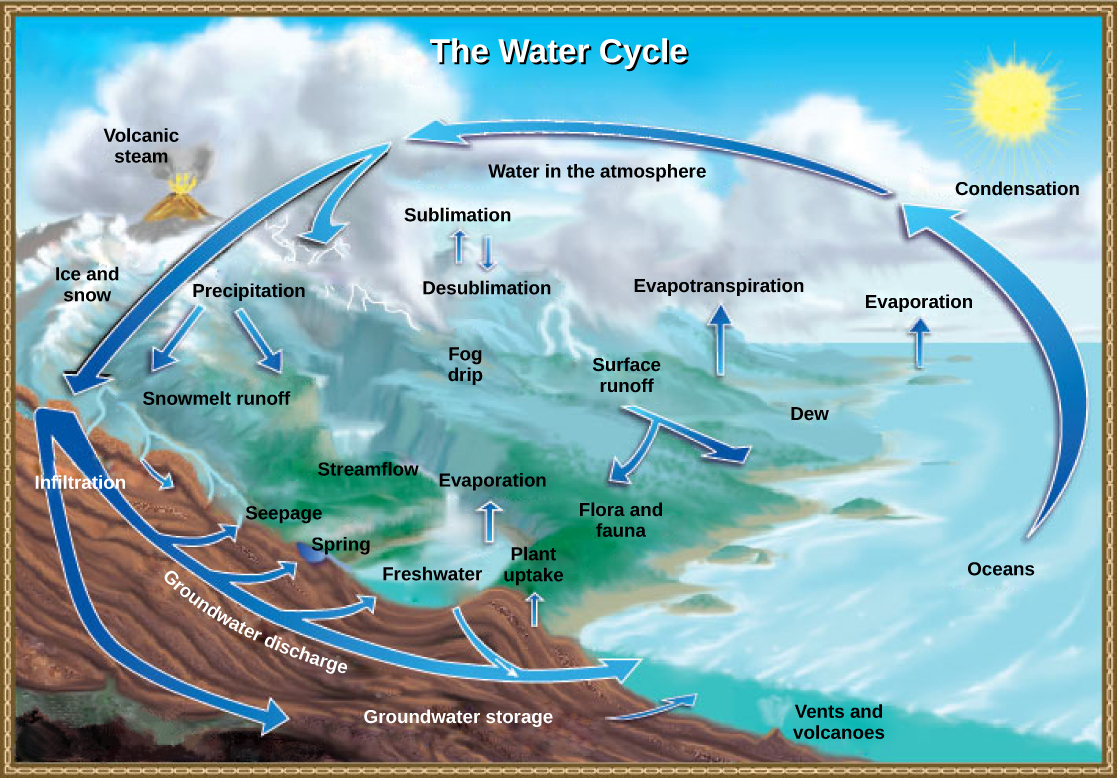
The carbon cycle is most easily studied as two interconnected subcycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes. The entire carbon cycle is shown in [link] .
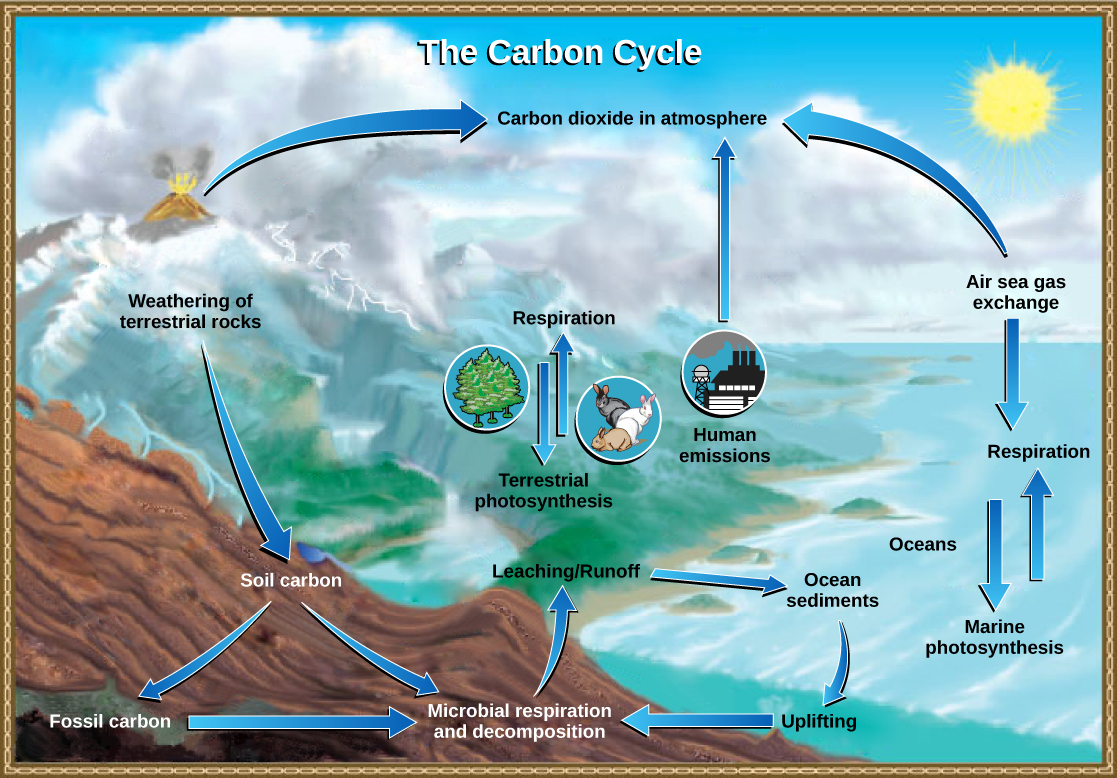
Living organisms are connected in many ways, even between ecosystems. A good example of this connection is the exchange of carbon between heterotrophs and autotrophs within and between ecosystems by way of atmospheric carbon dioxide. Carbon dioxide is the basic building block that autotrophs use to build multi-carbon, high-energy compounds, such as glucose. The energy harnessed from the Sun is used by these organisms to form the covalent bonds that link carbon atoms together. These chemical bonds store this energy for later use in the process of respiration. Most terrestrial autotrophs obtain their carbon dioxide directly from the atmosphere, while marine autotrophs acquire it in the dissolved form (carbonic acid, HCO 3 – ). However the carbon dioxide is acquired, a byproduct of fixing carbon in organic compounds is oxygen. Photosynthetic organisms are responsible for maintaining approximately 21 percent of the oxygen content of the atmosphere that we observe today.
The partners in biological carbon exchange are the heterotrophs (especially the primary consumers, largely herbivores). Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them and breaking them down by respiration to obtain cellular energy, such as ATP. The most efficient type of respiration, aerobic respiration, requires oxygen obtained from the atmosphere or dissolved in water. Thus, there is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Autotrophs also respire and consume the organic molecules they form: using oxygen and releasing carbon dioxide. They release more oxygen gas as a waste product of photosynthesis than they use for their own respiration; therefore, there is excess available for the respiration of other aerobic organisms. Gas exchange through the atmosphere and water is one way that the carbon cycle connects all living organisms on Earth.

Notification Switch
Would you like to follow the 'Concepts of biology' conversation and receive update notifications?