| << Chapter < Page | Chapter >> Page > |
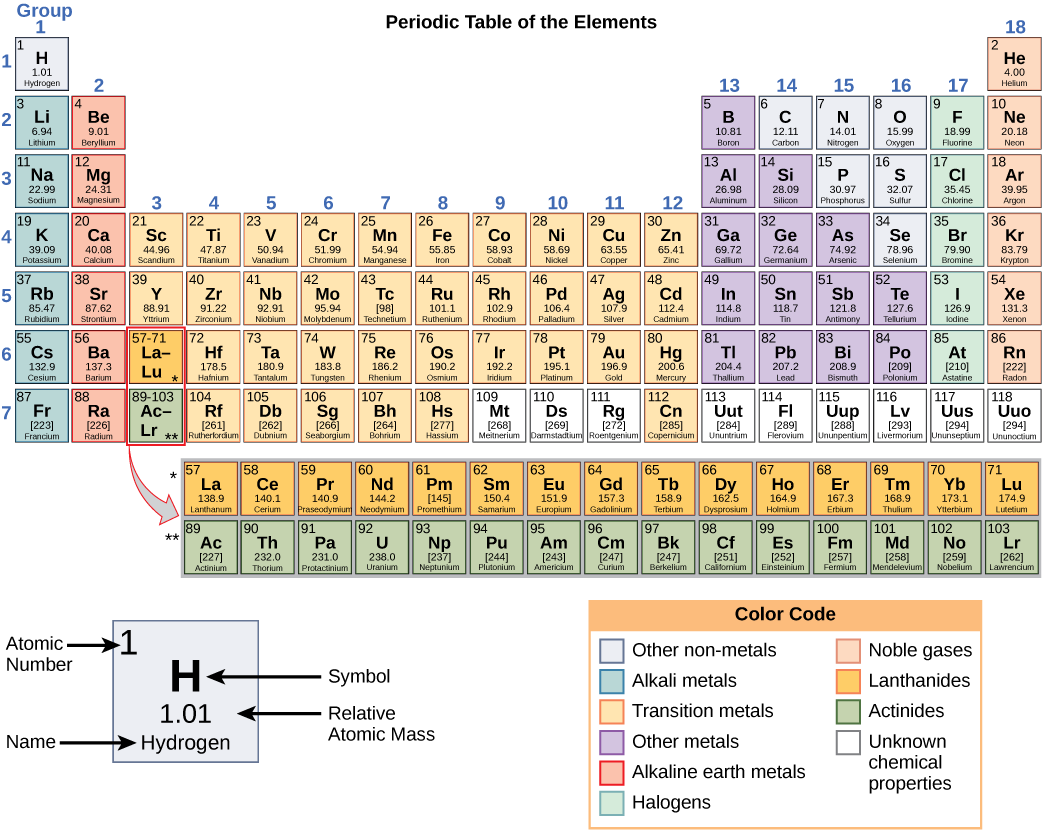
These numbers provide information about the elements and how they will react when combined. Different elements have different melting and boiling points, and are in different states (liquid, solid, or gas) at room temperature. They also combine in different ways. Some form specific types of bonds, whereas others do not. How they combine is based on the number of electrons present. Because of these characteristics, the elements are arranged into the periodic table of elements , a chart of the elements that includes the atomic number and relative atomic mass of each element. The periodic table also provides key information about the properties of elements ( [link] )—often indicated by color-coding. The arrangement of the table also shows how the electrons in each element are organized and provides important details about how atoms will react with each other to form molecules.
Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon-12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons and six neutrons) and an atomic number of 6 (which makes it carbon). Carbon-14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons and eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes.
How many neutrons do (K) potassium-39 and potassium-40 have, respectively?
After approximately 5,730 years, only one-half of the starting concentration of 14 C will have been converted to 14 N. The time it takes for half of the original concentration of an isotope to decay to its more stable form is called its half-life. Because the half-life of 14 C is long, it is used to age formerly living objects, such as fossils. Using the ratio of the 14 C concentration found in an object to the amount of 14 C detected in the atmosphere, the amount of the isotope that has not yet decayed can be determined. Based on this amount, the age of the fossil can be calculated to about 50,000 years ( [link] ). Isotopes with longer half-lives, such as potassium-40, are used to calculate the ages of older fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years.


Notification Switch
Would you like to follow the 'Concepts of biology' conversation and receive update notifications?