| << Chapter < Page | Chapter >> Page > |
Phosphorus is an essential nutrient for living processes; it is a major component of nucleic acid and phospholipids, and, as calcium phosphate, makes up the supportive components of our bones. Phosphorus is often the limiting nutrient (necessary for growth) in aquatic ecosystems ( [link] ).
Phosphorus occurs in nature as the phosphate ion (PO 4 3− ). In addition to phosphate runoff as a result of human activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the ocean. This rock has its origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, in remote regions, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of areas of the Earth’s surface.
Phosphorus is also reciprocally exchanged between phosphate dissolved in the ocean and marine ecosystems. The movement of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.
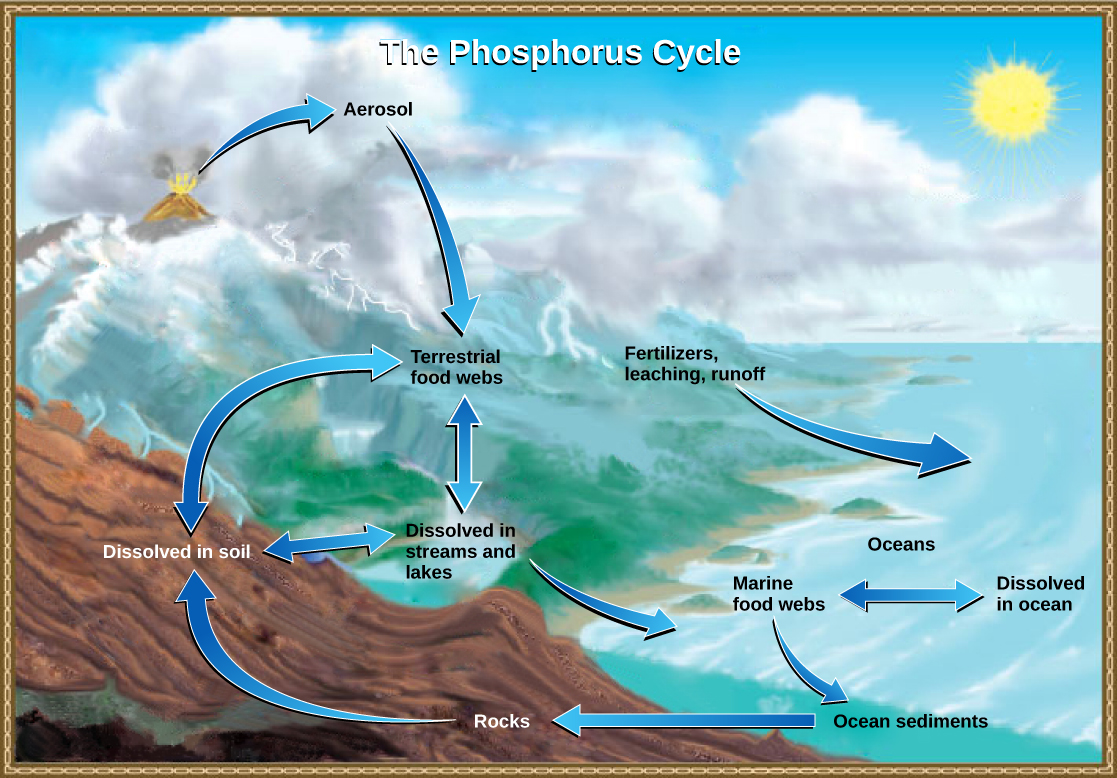
Excess phosphorus and nitrogen that enters these ecosystems from fertilizer runoff and from sewage causes excessive growth of microorganisms and depletes the dissolved oxygen, which leads to the death of many ecosystem fauna, such as shellfish and finfish. This process is responsible for dead zones in lakes and at the mouths of many major rivers ( [link] ).
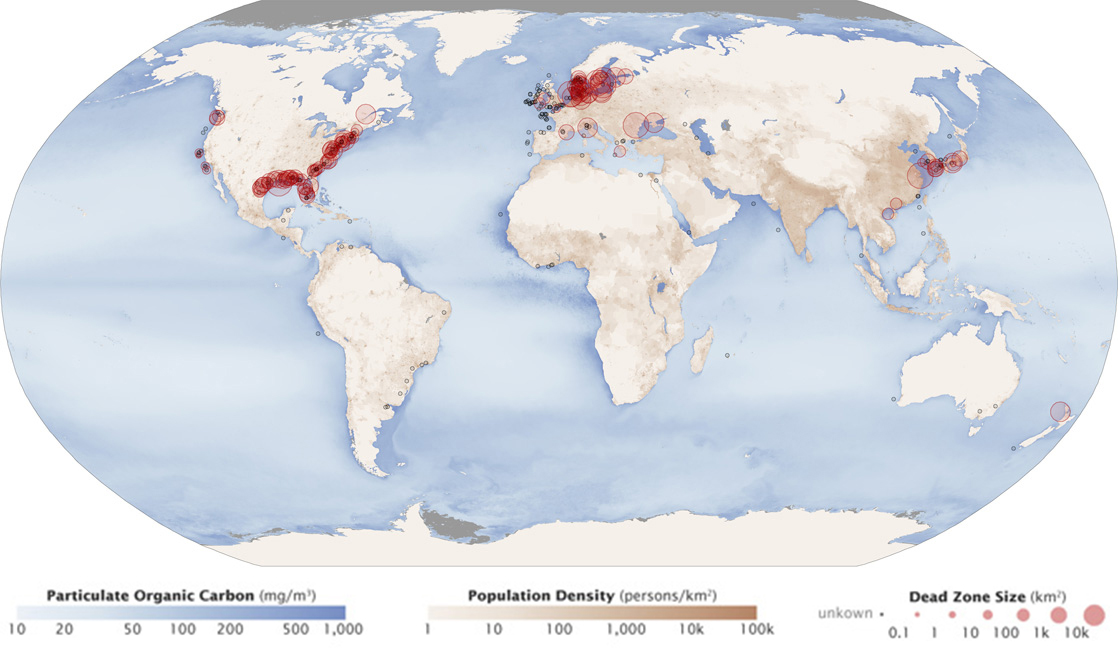
A dead zone is an area within a freshwater or marine ecosystem where large areas are depleted of their normal flora and fauna; these zones can be caused by eutrophication, oil spills, dumping of toxic chemicals, and other human activities. The number of dead zones has been increasing for several years, and more than 400 of these zones were present as of 2008. One of the worst dead zones is off the coast of the United States in the Gulf of Mexico, where fertilizer runoff from the Mississippi River basin has created a dead zone of over 8463 square miles. Phosphate and nitrate runoff from fertilizers also negatively affect several lake and bay ecosystems including the Chesapeake Bay in the eastern United States.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?