| << Chapter < Page | Chapter >> Page > |

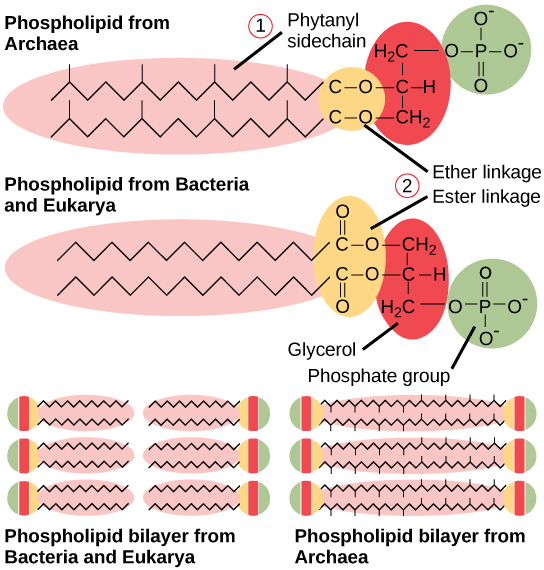
The plasma membrane is a thin lipid bilayer (6 to 8 nanometers) that completely surrounds the cell and separates the inside from the outside. Its selectively permeable nature keeps ions, proteins, and other molecules within the cell and prevents them from diffusing into the extracellular environment, while other molecules may move through the membrane. Recall that the general structure of a cell membrane is a phospholipid bilayer composed of two layers of lipid molecules. In archaeal cell membranes, isoprene (phytanyl) chains linked to glycerol replace the fatty acids linked to glycerol in bacterial membranes. Some archaeal membranes are lipid monolayers instead of bilayers ( [link] ).
The cytoplasm of prokaryotic cells has a high concentration of dissolved solutes. Therefore, the osmotic pressure within the cell is relatively high. The cell wall is a protective layer that surrounds some cells and gives them shape and rigidity. It is located outside the cell membrane and prevents osmotic lysis (bursting due to increasing volume). The chemical composition of the cell walls varies between archaea and bacteria, and also varies between bacterial species.
Bacterial cell walls contain peptidoglycan , composed of polysaccharide chains that are cross-linked by unusual peptides containing both L- and D-amino acids including D-glutamic acid and D-alanine. Proteins normally have only L-amino acids; as a consequence, many of our antibiotics work by mimicking D-amino acids and therefore have specific effects on bacterial cell wall development. There are more than 100 different forms of peptidoglycan. S-layer (surface layer) proteins are also present on the outside of cell walls of both archaea and bacteria.
Bacteria are divided into two major groups: Gram positive and Gram negative , based on their reaction to Gram staining. Note that all Gram-positive bacteria belong to one phylum; bacteria in the other phyla (Proteobacteria, Chlamydias, Spirochetes, Cyanobacteria, and others) are Gram-negative. The Gram staining method is named after its inventor, Danish scientist Hans Christian Gram (1853–1938). The different bacterial responses to the staining procedure are ultimately due to cell wall structure. Gram-positive organisms typically lack the outer membrane found in Gram-negative organisms ( [link] ). Up to 90 percent of the cell wall in Gram-positive bacteria is composed of peptidoglycan, and most of the rest is composed of acidic substances called teichoic acids . Teichoic acids may be covalently linked to lipids in the plasma membrane to form lipoteichoic acids. Lipoteichoic acids anchor the cell wall to the cell membrane. Gram-negative bacteria have a relatively thin cell wall composed of a few layers of peptidoglycan (only 10 percent of the total cell wall), surrounded by an outer envelope containing lipopolysaccharides (LPS) and lipoproteins. This outer envelope is sometimes referred to as a second lipid bilayer. The chemistry of this outer envelope is very different, however, from that of the typical lipid bilayer that forms plasma membranes.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?