| << Chapter < Page | Chapter >> Page > |
The centrosome (the organelle where all microtubules originate) replicates itself before a cell divides, and the centrioles appear to have some role in pulling the duplicated chromosomes to opposite ends of the dividing cell. However, the exact function of the centrioles in cell division isn’t clear, because cells that have had the centrosome removed can still divide, and plant cells, which lack centrosomes, are capable of cell division.
Animal cells have another set of organelles not found in plant cells: lysosomes. The lysosomes are the cell’s “garbage disposal.” In plant cells, the digestive processes take place in vacuoles. Enzymes within the lysosomes aid the breakdown of proteins, polysaccharides, lipids, nucleic acids, and even worn-out organelles. These enzymes are active at a much lower pH than that of the cytoplasm. Therefore, the pH within lysosomes is more acidic than the pH of the cytoplasm. Many reactions that take place in the cytoplasm could not occur at a low pH, so again, the advantage of compartmentalizing the eukaryotic cell into organelles is apparent.
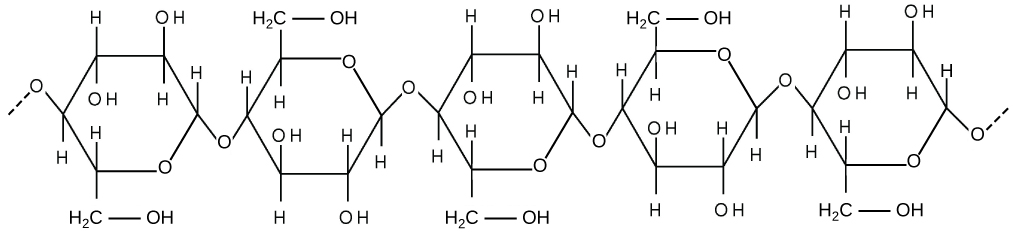
If you examine [link] b , the diagram of a plant cell, you will see a structure external to the plasma membrane called the cell wall. The cell wall is a rigid covering that protects the cell, provides structural support, and gives shape to the cell. Fungal and protistan cells also have cell walls. While the chief component of prokaryotic cell walls is peptidoglycan, the major organic molecule in the plant cell wall is cellulose ( [link] ), a polysaccharide made up of glucose units. Have you ever noticed that when you bite into a raw vegetable, like celery, it crunches? That’s because you are tearing the rigid cell walls of the celery cells with your teeth.
Like the mitochondria, chloroplasts have their own DNA and ribosomes, but chloroplasts have an entirely different function. Chloroplasts are plant cell organelles that carry out photosynthesis. Photosynthesis is the series of reactions that use carbon dioxide, water, and light energy to make glucose and oxygen. This is a major difference between plants and animals; plants (autotrophs) are able to make their own food, like sugars, while animals (heterotrophs) must ingest their food.
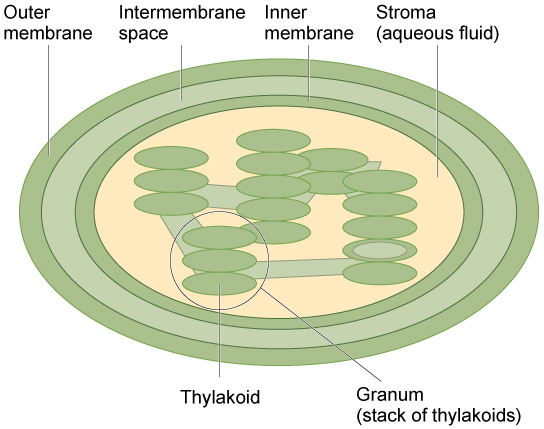
Like mitochondria, chloroplasts have outer and inner membranes, but within the space enclosed by a chloroplast’s inner membrane is a set of interconnected and stacked fluid-filled membrane sacs called thylakoids ( [link] ). Each stack of thylakoids is called a granum (plural = grana). The fluid enclosed by the inner membrane that surrounds the grana is called the stroma.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?