| << Chapter < Page | Chapter >> Page > |
In aqueous environments and their anoxic sediments, there is another carbon cycle taking place. In this case, the cycle is based on one-carbon compounds. In anoxic sediments, prokaryotes, mostly archaea, produce methane (CH 4 ). This methane moves into the zone above the sediment, which is richer in oxygen and supports bacteria called methane oxidizers that oxidize methane to carbon dioxide, which then returns to the atmosphere.
Nitrogen is a very important element for life because it is part of proteins and nucleic acids. It is a macronutrient, and in nature, it is recycled from organic compounds to ammonia, ammonium ions, nitrate, nitrite, and nitrogen gas by myriad processes, many of which are carried out only by prokaryotes. As illustrated in [link] , prokaryotes are key to the nitrogen cycle. The largest pool of nitrogen available in the terrestrial ecosystem is gaseous nitrogen from the air, but this nitrogen is not usable by plants, which are primary producers. Gaseous nitrogen is transformed, or “fixed” into more readily available forms such as ammonia through the process of nitrogen fixation . Ammonia can be used by plants or converted to other forms.
Another source of ammonia is ammonification , the process by which ammonia is released during the decomposition of nitrogen-containing organic compounds. Ammonia released to the atmosphere, however, represents only 15 percent of the total nitrogen released; the rest is as N 2 and N 2 O. Ammonia is catabolized anaerobically by some prokaryotes, yielding N 2 as the final product. Nitrification is the conversion of ammonium to nitrite and nitrate. Nitrification in soils is carried out by bacteria belonging to the genera Nitrosomas , Nitrobacter , and Nitrospira . The bacteria performs the reverse process, the reduction of nitrate from the soils to gaseous compounds such as N 2 O, NO, and N 2 , a process called denitrification .
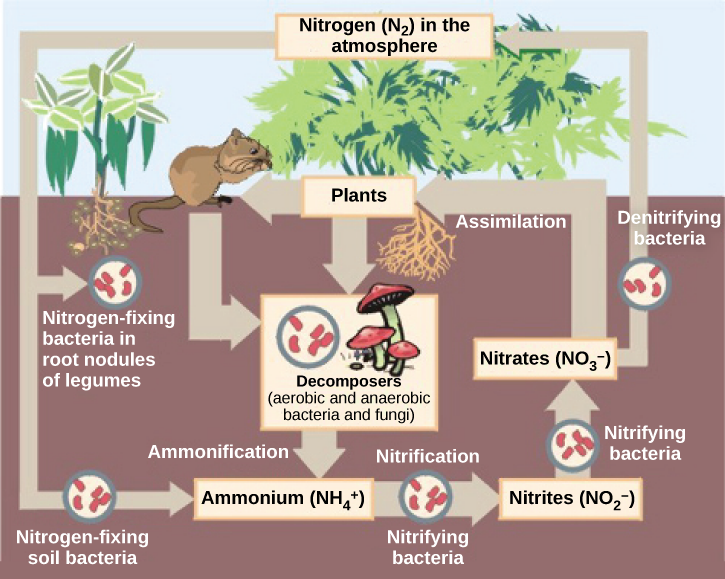
Which of the following statements about the nitrogen cycle is false?
Prokaryotes are the most metabolically diverse organisms; they flourish in many different environments with various carbon energy and carbon sources, variable temperature, pH, pressure, and water availability. Nutrients required in large amounts are called macronutrients, whereas those required in trace amounts are called micronutrients or trace elements. Macronutrients include C, H, O, N, P, S, K, Mg, Ca, and Na. In addition to these macronutrients, prokaryotes require various metallic elements for growth and enzyme function. Prokaryotes use different sources of energy to assemble macromolecules from smaller molecules. Phototrophs obtain their energy from sunlight, whereas chemotrophs obtain energy from chemical compounds.
Prokaryotes play roles in the carbon and nitrogen cycles. Carbon is returned to the atmosphere by the respiration of animals and other chemoorganotrophic organisms. Consumers use organic compounds generated by producers and release carbon dioxide into the atmosphere. The most important contributor of carbon dioxide to the atmosphere is microbial decomposition of dead material. Nitrogen is recycled in nature from organic compounds to ammonia, ammonium ions, nitrite, nitrate, and nitrogen gas. Gaseous nitrogen is transformed into ammonia through nitrogen fixation. Ammonia is anaerobically catabolized by some prokaryotes, yielding N 2 as the final product. Nitrification is the conversion of ammonium into nitrite. Nitrification in soils is carried out by bacteria. Denitrification is also performed by bacteria and transforms nitrate from soils into gaseous nitrogen compounds, such as N 2 O, NO, and N 2 .
[link] Which of the following statements about the nitrogen cycle is false?
[link] D

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?