| << Chapter < Page | Chapter >> Page > |
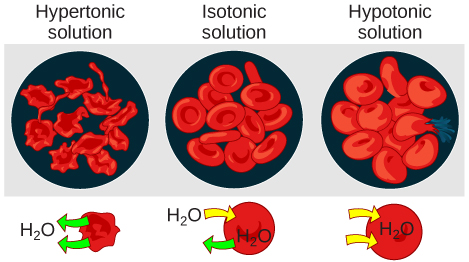
As for a hypertonic solution, the prefix hyper - refers to the extracellular fluid having a higher osmolarity than the cell’s cytoplasm; therefore, the fluid contains less water than the cell does. Because the cell has a relatively higher concentration of water, water will leave the cell.
In an isotonic solution, the extracellular fluid has the same osmolarity as the cell. If the osmolarity of the cell matches that of the extracellular fluid, there will be no net movement of water into or out of the cell, although water will still move in and out. Blood cells and plant cells in hypertonic, isotonic, and hypotonic solutions take on characteristic appearances ( [link] ).
A doctor injects a patient with what the doctor thinks is an isotonic saline solution. The patient dies, and an autopsy reveals that many red blood cells have been destroyed. Do you think the solution the doctor injected was really isotonic?
For a video illustrating the process of diffusion in solutions, visit this site .
In a hypotonic environment, water enters a cell, and the cell swells. In an isotonic condition, the relative concentrations of solute and solvent are equal on both sides of the membrane. There is no net water movement; therefore, there is no change in the size of the cell. In a hypertonic solution, water leaves a cell and the cell shrinks. If either the hypo- or hyper- condition goes to excess, the cell’s functions become compromised, and the cell may be destroyed.
A red blood cell will burst, or lyse, when it swells beyond the plasma membrane’s capability to expand. Remember, the membrane resembles a mosaic, with discrete spaces between the molecules composing it. If the cell swells, and the spaces between the lipids and proteins become too large, the cell will break apart.
In contrast, when excessive amounts of water leave a red blood cell, the cell shrinks, or crenates. This has the effect of concentrating the solutes left in the cell, making the cytosol denser and interfering with diffusion within the cell. The cell’s ability to function will be compromised and may also result in the death of the cell.
Various living things have ways of controlling the effects of osmosis—a mechanism called osmoregulation. Some organisms, such as plants, fungi, bacteria, and some protists, have cell walls that surround the plasma membrane and prevent cell lysis in a hypotonic solution. The plasma membrane can only expand to the limit of the cell wall, so the cell will not lyse. In fact, the cytoplasm in plants is always slightly hypertonic to the cellular environment, and water will always enter a cell if water is available. This inflow of water produces turgor pressure, which stiffens the cell walls of the plant ( [link] ). In nonwoody plants, turgor pressure supports the plant. Conversly, if the plant is not watered, the extracellular fluid will become hypertonic, causing water to leave the cell. In this condition, the cell does not shrink because the cell wall is not flexible. However, the cell membrane detaches from the wall and constricts the cytoplasm. This is called plasmolysis . Plants lose turgor pressure in this condition and wilt ( [link] ).

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?