| << Chapter < Page | Chapter >> Page > |
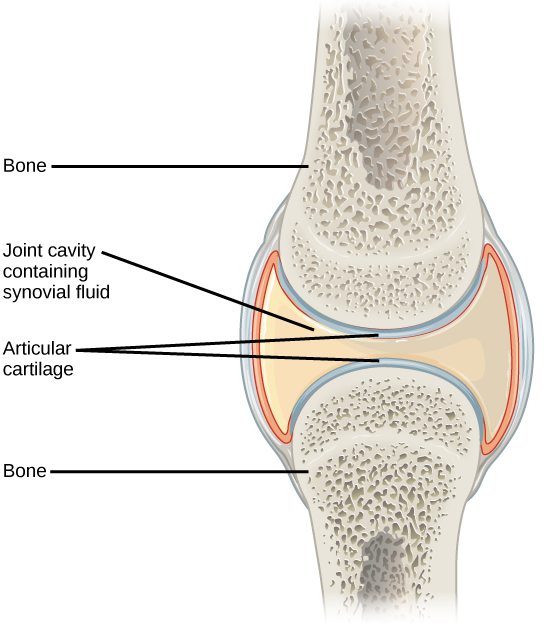
The functional classification divides joints into three categories: synarthroses, amphiarthroses, and diarthroses. A synarthrosis is a joint that is immovable. This includes sutures, gomphoses, and synchondroses. Amphiarthroses are joints that allow slight movement, including syndesmoses and symphyses. Diarthroses are joints that allow for free movement of the joint, as in synovial joints.
The wide range of movement allowed by synovial joints produces different types of movements. The movement of synovial joints can be classified as one of four different types: gliding, angular, rotational, or special movement.
Gliding movements occur as relatively flat bone surfaces move past each other. Gliding movements produce very little rotation or angular movement of the bones. The joints of the carpal and tarsal bones are examples of joints that produce gliding movements.
Angular movements are produced when the angle between the bones of a joint changes. There are several different types of angular movements, including flexion, extension, hyperextension, abduction, adduction, and circumduction. Flexion , or bending, occurs when the angle between the bones decreases. Moving the forearm upward at the elbow or moving the wrist to move the hand toward the forearm are examples of flexion. Extension is the opposite of flexion in that the angle between the bones of a joint increases. Straightening a limb after flexion is an example of extension. Extension past the regular anatomical position is referred to as hyperextension . This includes moving the neck back to look upward, or bending the wrist so that the hand moves away from the forearm.
Abduction occurs when a bone moves away from the midline of the body. Examples of abduction are moving the arms or legs laterally to lift them straight out to the side. Adduction is the movement of a bone toward the midline of the body. Movement of the limbs inward after abduction is an example of adduction. Circumduction is the movement of a limb in a circular motion, as in moving the arm in a circular motion.
Rotational movement is the movement of a bone as it rotates around its longitudinal axis. Rotation can be toward the midline of the body, which is referred to as medial rotation , or away from the midline of the body, which is referred to as lateral rotation . Movement of the head from side to side is an example of rotation.
Some movements that cannot be classified as gliding, angular, or rotational are called special movements. Inversion involves the soles of the feet moving inward, toward the midline of the body. Eversion is the opposite of inversion, movement of the sole of the foot outward, away from the midline of the body. Protraction is the anterior movement of a bone in the horizontal plane. Retraction occurs as a joint moves back into position after protraction. Protraction and retraction can be seen in the movement of the mandible as the jaw is thrust outwards and then back inwards. Elevation is the movement of a bone upward, such as when the shoulders are shrugged, lifting the scapulae. Depression is the opposite of elevation—movement downward of a bone, such as after the shoulders are shrugged and the scapulae return to their normal position from an elevated position. Dorsiflexion is a bending at the ankle such that the toes are lifted toward the knee. Plantar flexion is a bending at the ankle when the heel is lifted, such as when standing on the toes. Supination is the movement of the radius and ulna bones of the forearm so that the palm faces forward. Pronation is the opposite movement, in which the palm faces backward. Opposition is the movement of the thumb toward the fingers of the same hand, making it possible to grasp and hold objects.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?