| << Chapter < Page | Chapter >> Page > |
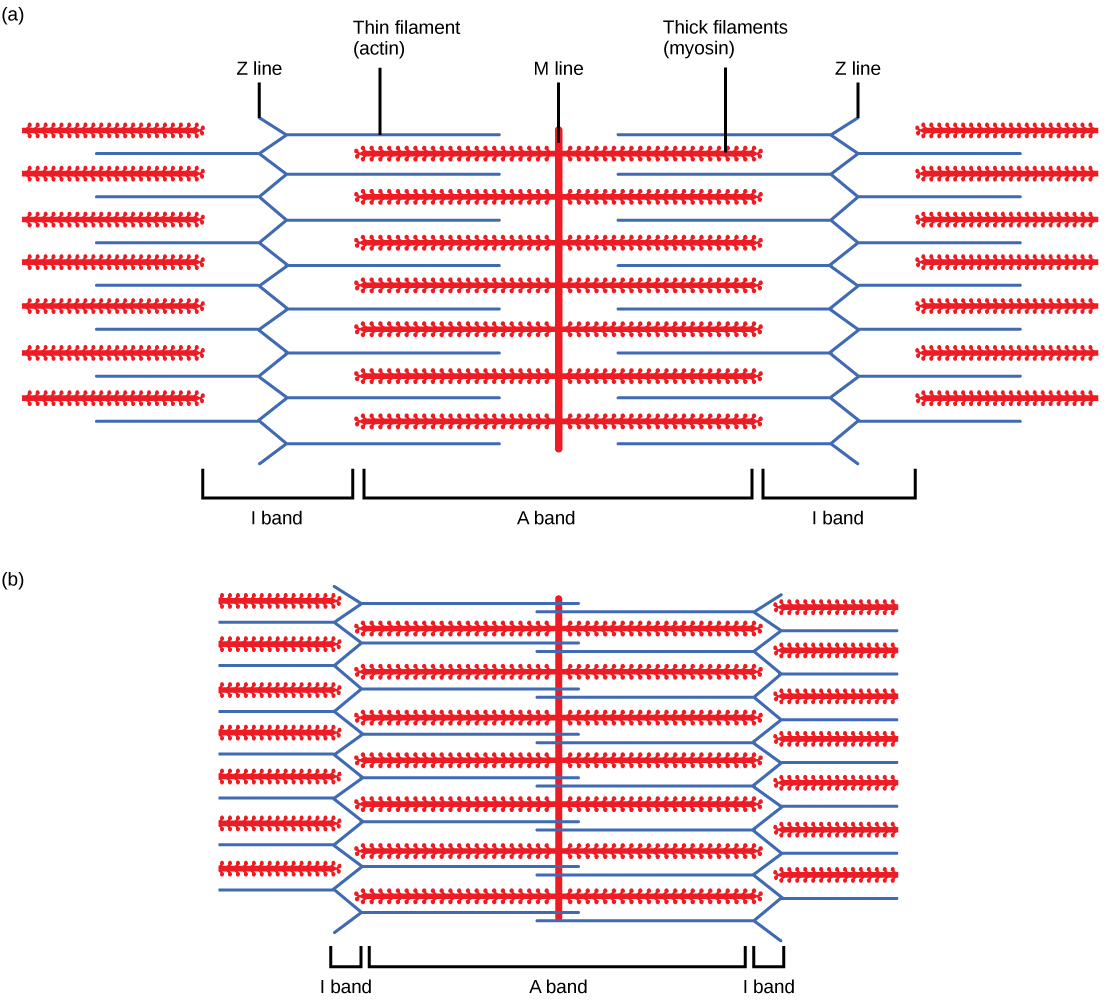
When a sarcomere shortens, some regions shorten whereas others stay the same length. A sarcomere is defined as the distance between two consecutive Z discs or Z lines; when a muscle contracts, the distance between the Z discs is reduced. The H zone—the central region of the A zone—contains only thick filaments and is shortened during contraction. The I band contains only thin filaments and also shortens. The A band does not shorten—it remains the same length—but A bands of different sarcomeres move closer together during contraction, eventually disappearing. Thin filaments are pulled by the thick filaments toward the center of the sarcomere until the Z discs approach the thick filaments. The zone of overlap, in which thin filaments and thick filaments occupy the same area, increases as the thin filaments move inward.
The motion of muscle shortening occurs as myosin heads bind to actin and pull the actin inwards. This action requires energy, which is provided by ATP. Myosin binds to actin at a binding site on the globular actin protein. Myosin has another binding site for ATP at which enzymatic activity hydrolyzes ATP to ADP, releasing an inorganic phosphate molecule and energy.
ATP binding causes myosin to release actin, allowing actin and myosin to detach from each other. After this happens, the newly bound ATP is converted to ADP and inorganic phosphate, P i . The enzyme at the binding site on myosin is called ATPase. The energy released during ATP hydrolysis changes the angle of the myosin head into a “cocked” position. The myosin head is then in a position for further movement, possessing potential energy, but ADP and P i are still attached. If actin binding sites are covered and unavailable, the myosin will remain in the high energy configuration with ATP hydrolyzed, but still attached.
If the actin binding sites are uncovered, a cross-bridge will form; that is, the myosin head spans the distance between the actin and myosin molecules. P i is then released, allowing myosin to expend the stored energy as a conformational change. The myosin head moves toward the M line, pulling the actin along with it. As the actin is pulled, the filaments move approximately 10 nm toward the M line. This movement is called the power stroke, as it is the step at which force is produced. As the actin is pulled toward the M line, the sarcomere shortens and the muscle contracts.
When the myosin head is “cocked,” it contains energy and is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke; at the end of the power stroke, the myosin head is in a low-energy position. After the power stroke, ADP is released; however, the cross-bridge formed is still in place, and actin and myosin are bound together. ATP can then attach to myosin, which allows the cross-bridge cycle to start again and further muscle contraction can occur ( [link] ).

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?