| << Chapter < Page | Chapter >> Page > |
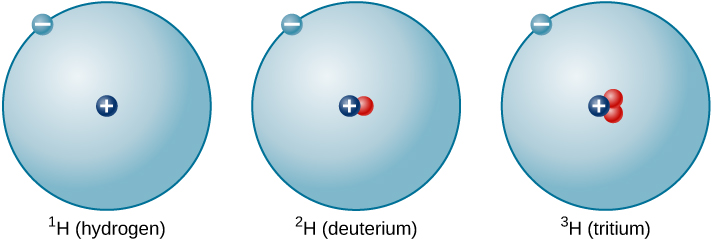
The ratio of neutrons to protons increases as the number of protons increases, but each element is unique. The number of neutrons is not necessarily the same for all atoms of a given element. For example, most hydrogen atoms contain no neutrons at all. There are, however, hydrogen atoms that contain one proton and one neutron, and others that contain one proton and two neutrons. The various types of hydrogen nuclei with different numbers of neutrons are called isotopes of hydrogen ( [link] ), and all other elements have isotopes as well. You can think of isotopes as siblings in the same element “family”—closely related but with different characteristics and behaviors.
To explore the structure of atoms, go to the PhET Build and Atom website where you can add protons, neutrons, or electrons to a model and the name of the element you have created will appear. You can also see the net charge, the mass number, whether it is stable or unstable, and whether it is an ion or a neutral atom.
Rutherford’s model for atoms has one serious problem. Maxwell’s theory of electromagnetic radiation says that when electrons change either speed or the direction of motion, they must emit energy. Orbiting electrons constantly change their direction of motion, so they should emit a constant stream of energy. Applying Maxwell’s theory to Rutherford’s model, all electrons should spiral into the nucleus of the atom as they lose energy, and this collapse should happen very quickly—in about 10 –16 seconds.
It was Danish physicist Niels Bohr (1885–1962) who solved the mystery of how electrons remain in orbit. He was trying to develop a model of the atom that would also explain certain regularities observed in the spectrum of hydrogen. He suggested that the spectrum of hydrogen can be understood if we assume that orbits of only certain sizes are possible for the electron. Bohr further assumed that as long as the electron moves in only one of these allowed orbits, it radiates no energy: its energy would change only if it moved from one orbit to another.
This suggestion, in the words of science historian Abraham Pais, was “one of the most audacious hypotheses ever introduced in physics.” If something equivalent were at work in the everyday world, you might find that, as you went for a walk after astronomy class, nature permitted you to walk two steps per minute, five steps per minute, and 12 steps per minute, but no speeds in between. No matter how you tried to move your legs, only certain walking speeds would be permitted. To make things more bizarre, it would take no effort to walk at any one of the allowed speeds, but it would be difficult to change from one speed to another. Luckily, no such rules apply at the level of human behavior. But at the microscopic level of the atom, experiment after experiment has confirmed the validity of Bohr’s strange idea. Bohr’s suggestions became one of the foundations of the new (and much more sophisticated) model of the subatomic world called quantum mechanics.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?