| << Chapter < Page | Chapter >> Page > |
But the crucial way that massive stars diverge from the story we have outlined is that they can start additional kinds of fusion in their centers and in the shells surrounding their central regions. The outer layers of a star with a mass greater than about 8 solar masses have a weight that is enough to compress the carbon-oxygen core until it becomes hot enough to ignite fusion of carbon nuclei. Carbon can fuse into still more oxygen, and at still higher temperatures, oxygen and then neon, magnesium, and finally silicon can build even heavier elements. Iron is, however, the endpoint of this process. The fusion of iron atoms produces products that are more massive than the nuclei that are being fused and therefore the process requires energy, as opposed to releasing energy, which all fusion reactions up to this point have done. This required energy comes at the expense of the star itself, which is now on the brink of death ( [link] ). What happens next will be described in the chapter on The Death of Stars .
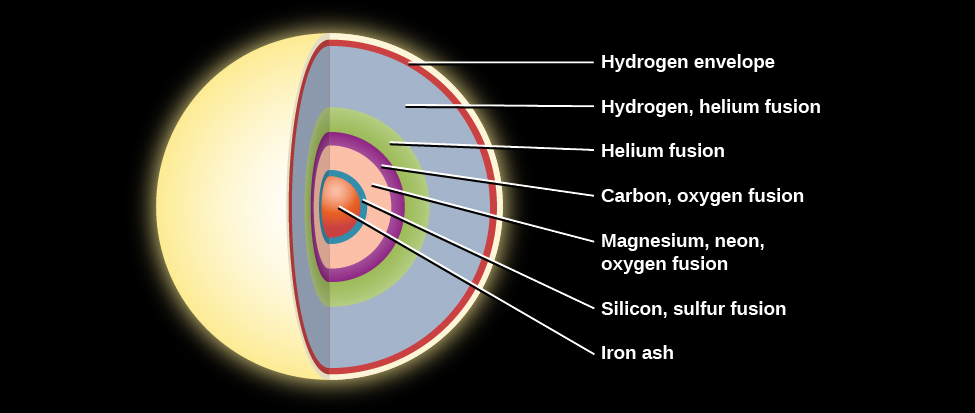
Physicists have now found nuclear pathways whereby virtually all chemical elements of atomic weights up to that of iron can be built up by this nucleosynthesis (the making of new atomic nuclei) in the centers of the more massive red giant stars. This still leaves the question of where elements heavier than iron come from. We will see in the next chapter that when massive stars finally exhaust their nuclear fuel, they most often die in a spectacular explosion—a supernova. Heavier elements can be synthesized in the stunning violence of such explosions.
Not only can we explain in this way where the elements that make up our world and others come from, but our theories of nucleosynthesis inside stars are even able to predict the relative abundances with which the elements occur in nature. The way stars build up elements during various nuclear reactions really can explain why some elements (oxygen, carbon, and iron) are common and others are quite rare (gold, silver, and uranium).
The fact that the elements are made in stars over time explains an important difference between globular and open clusters. Hydrogen and helium, which are the most abundant elements in stars in the solar neighborhood, are also the most abundant constituents of stars in both kinds of clusters. However, the abundances of the elements heavier than helium are very different.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?