| << Chapter < Page | Chapter >> Page > |
Atoms that have absorbed specific photons from a passing beam of white light and have thus become excited generally de-excite themselves and emit that light again in a very short time. You might wonder, then, why dark spectral lines are ever produced. In other words, why doesn’t this reemitted light quickly “fill in” the darker absorption lines?
Imagine a beam of white light coming toward you through some cooler gas. Some of the reemitted light is actually returned to the beam of white light you see, but this fills in the absorption lines only to a slight extent. The reason is that the atoms in the gas reemit light in all directions , and only a small fraction of the reemitted light is in the direction of the original beam (toward you). In a star, much of the reemitted light actually goes in directions leading back into the star, which does observers outside the star no good whatsoever.
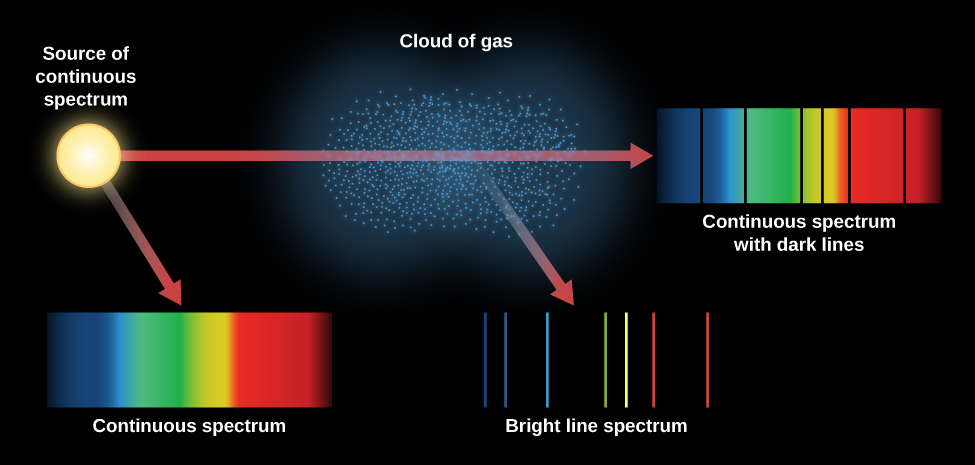
[link] summarizes the different kinds of spectra we have discussed. An incandescent lightbulb produces a continuous spectrum. When that continuous spectrum is viewed through a thinner cloud of gas, an absorption line spectrum can be seen superimposed on the continuous spectrum. If we look only at a cloud of excited gas atoms (with no continuous source seen behind it), we see that the excited atoms give off an emission line spectrum.
Atoms in a hot gas are moving at high speeds and continually colliding with one another and with any loose electrons. They can be excited (electrons moving to a higher level) and de-excited (electrons moving to a lower level) by these collisions as well as by absorbing and emitting light. The speed of atoms in a gas depends on the temperature. When the temperature is higher, so are the speed and energy of the collisions. The hotter the gas, therefore, the more likely that electrons will occupy the outermost orbits, which correspond to the highest energy levels. This means that the level where electrons start their upward jumps in a gas can serve as an indicator of how hot that gas is. In this way, the absorption lines in a spectrum give astronomers information about the temperature of the regions where the lines originate.
Use this simulation to play with a hydrogen atom and see what happens when electrons move to higher levels and then give off photons as they go to a lower level.
We have described how certain discrete amounts of energy can be absorbed by an atom, raising it to an excited state and moving one of its electrons farther from its nucleus. If enough energy is absorbed, the electron can be completely removed from the atom—this is called ionization . The atom is then said to be ionized. The minimum amount of energy required to remove one electron from an atom in its ground state is called its ionization energy.
Still-greater amounts of energy must be absorbed by the now-ionized atom (called an ion ) to remove an additional electron deeper in the structure of the atom. Successively greater energies are needed to remove the third, fourth, fifth—and so on—electrons from the atom. If enough energy is available, an atom can become completely ionized, losing all of its electrons. A hydrogen atom, having only one electron to lose, can be ionized only once; a helium atom can be ionized twice; and an oxygen atom up to eight times. When we examine regions of the cosmos where there is a great deal of energetic radiation, such as the neighborhoods where hot young stars have recently formed, we see a lot of ionization going on.
An atom that has become positively ionized has lost a negative charge—the missing electron—and thus is left with a net positive charge. It therefore exerts a strong attraction on any free electron. Eventually, one or more electrons will be captured and the atom will become neutral (or ionized to one less degree) again. During the electron-capture process, the atom emits one or more photons. Which photons are emitted depends on whether the electron is captured at once to the lowest energy level of the atom or stops at one or more intermediate levels on its way to the lowest available level.
Just as the excitation of an atom can result from a collision with another atom, ion, or electron (collisions with electrons are usually most important), so can ionization. The rate at which such collisional ionizations occur depends on the speeds of the atoms and hence on the temperature of the gas—the hotter the gas, the more of its atoms will be ionized.
The rate at which ions and electrons recombine also depends on their relative speeds—that is, on the temperature. In addition, it depends on the density of the gas: the higher the density, the greater the chance for recapture, because the different kinds of particles are crowded more closely together. From a knowledge of the temperature and density of a gas, it is possible to calculate the fraction of atoms that have been ionized once, twice, and so on. In the Sun, for example, we find that most of the hydrogen and helium atoms in its atmosphere are neutral, whereas most of the calcium atoms, as well as many other heavier atoms, are ionized once.
The energy levels of an ionized atom are entirely different from those of the same atom when it is neutral. Each time an electron is removed from the atom, the energy levels of the ion, and thus the wavelengths of the spectral lines it can produce, change. This helps astronomers differentiate the ions of a given element. Ionized hydrogen, having no electron, can produce no absorption lines.
When electrons move from a higher energy level to a lower one, photons are emitted, and an emission line can be seen in the spectrum. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Since each atom has its own characteristic set of energy levels, each is associated with a unique pattern of spectral lines. This allows astronomers to determine what elements are present in the stars and in the clouds of gas and dust among the stars. An atom in its lowest energy level is in the ground state. If an electron is in an orbit other than the least energetic one possible, the atom is said to be excited. If an atom has lost one or more electrons, it is called an ion and is said to be ionized. The spectra of different ions look different and can tell astronomers about the temperatures of the sources they are observing.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?