| << Chapter < Page | Chapter >> Page > |
Astronomers and physicists have worked hard to learn the lines that go with each element by studying the way atoms absorb and emit light in laboratories here on Earth. Then they can use this knowledge to identify the elements in celestial bodies. In this way, we now know the chemical makeup of not just any star, but even galaxies of stars so distant that their light started on its way to us long before Earth had even formed.
Bohr’s model of the hydrogen atom was a great step forward in our understanding of the atom. However, we know today that atoms cannot be represented by quite so simple a picture. For example, the concept of sharply defined electron orbits is not really correct; however, at the level of this introductory course, the notion that only certain discrete energies are allowable for an atom is very useful. The energy levels we have been discussing can be thought of as representing certain average distances of the electron’s possible orbits from the atomic nucleus.
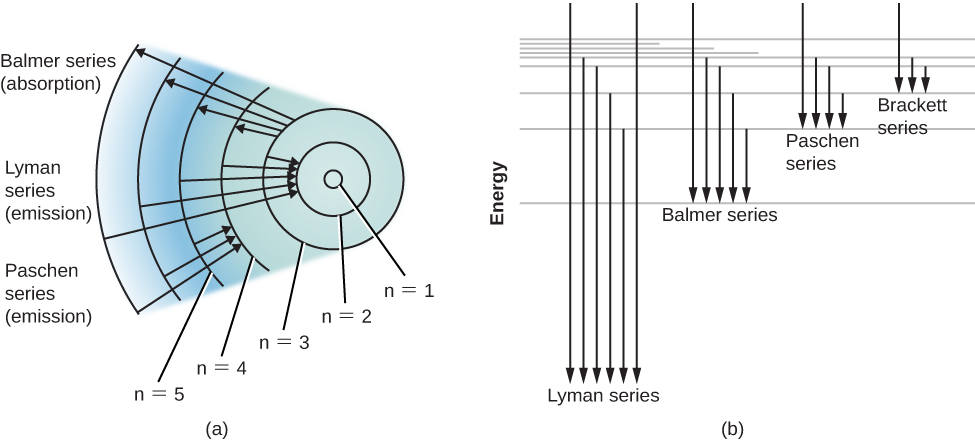
Ordinarily, an atom is in the state of lowest possible energy, its ground state . In the Bohr model of the hydrogen atom, the ground state corresponds to the electron being in the innermost orbit. An atom can absorb energy, which raises it to a higher energy level (corresponding, in the simple Bohr picture, to an electron’s movement to a larger orbit)—this is referred to as excitation . The atom is then said to be in an excited state . Generally, an atom remains excited for only a very brief time. After a short interval, typically a hundred-millionth of a second or so, it drops back spontaneously to its ground state, with the simultaneous emission of light. The atom may return to its lowest state in one jump, or it may make the transition in steps of two or more jumps, stopping at intermediate levels on the way down. With each jump, it emits a photon of the wavelength that corresponds to the energy difference between the levels at the beginning and end of that jump.
An energy-level diagram for a hydrogen atom and several possible atomic transitions are shown in [link] . When we measure the energies involved as the atom jumps between levels, we find that the transitions to or from the ground state, called the Lyman series of lines, result in the emission or absorption of ultraviolet photons. But the transitions to or from the first excited state (labeled n = 2 in part (a) of [link] ), called the Balmer series, produce emission or absorption in visible light. In fact, it was to explain this Balmer series that Bohr first suggested his model of the atom.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?