| << Chapter < Page | Chapter >> Page > |
Rutherford’s model required that the electrons be in motion. Positive and negative charges attract each other, so stationary electrons would fall into the positive nucleus. Also, because both the electrons and the nucleus are extremely small, most of the atom is empty, which is why nearly all of Rutherford’s particles were able to pass right through the gold foil without colliding with anything. Rutherford’s model was a very successful explanation of the experiments he conducted, although eventually scientists would discover that even the nucleus itself has structure.
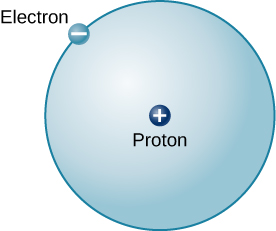
The simplest possible atom (and the most common one in the Sun and stars) is hydrogen. The nucleus of ordinary hydrogen contains a single proton. Moving around this proton is a single electron. The mass of an electron is nearly 2000 times smaller than the mass of a proton; the electron carries an amount of charge exactly equal to that of the proton but opposite in sign ( [link] ). Opposite charges attract each other, so it is an electromagnetic force that holds the proton and electron together, just as gravity is the force that keeps planets in orbit around the Sun.
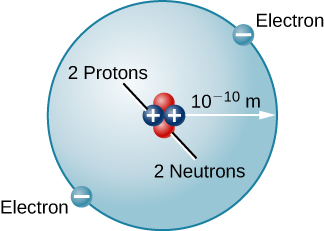
There are many other types of atoms in nature. Helium, for example, is the second-most abundant element in the Sun. Helium has two protons in its nucleus instead of the single proton that characterizes hydrogen. In addition, the helium nucleus contains two neutrons, particles with a mass comparable to that of the proton but with no electric charge. Moving around this nucleus are two electrons, so the total net charge of the helium atom is also zero ( [link] ).
From this description of hydrogen and helium, perhaps you have guessed the pattern for building up all the elements (different types of atoms) that we find in the universe. The type of element is determined by the number of protons in the nucleus of the atom. For example, any atom with six protons is the element carbon, with eight protons is oxygen, with 26 is iron, and with 92 is uranium. On Earth, a typical atom has the same number of electrons as protons, and these electrons follow complex orbital patterns around the nucleus. Deep inside stars, however, it is so hot that the electrons get loose from the nucleus and (as we shall see) lead separate yet productive lives.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?