| << Chapter < Page | Chapter >> Page > |
Scattered through the sea of exocrine acini are small islands of endocrine cells, the islets of Langerhans. These vital cells produce the hormones pancreatic polypeptide, insulin, glucagon, and somatostatin.
The pancreas produces over a liter of pancreatic juice each day. Unlike bile, it is clear and composed mostly of water along with some salts, sodium bicarbonate, and several digestive enzymes. Sodium bicarbonate is responsible for the slight alkalinity of pancreatic juice (pH 7.1 to 8.2), which serves to buffer the acidic gastric juice in chyme, inactivate pepsin from the stomach, and create an optimal environment for the activity of pH-sensitive digestive enzymes in the small intestine. Pancreatic enzymes are active in the digestion of sugars, proteins, and fats.
The pancreas produces protein-digesting enzymes in their inactive forms. These enzymes are activated in the duodenum. If produced in an active form, they would digest the pancreas (which is exactly what occurs in the disease, pancreatitis). The intestinal brush border enzyme enteropeptidase stimulates the activation of trypsin from trypsinogen of the pancreas, which in turn changes the pancreatic enzymes procarboxypeptidase and chymotrypsinogen into their active forms, carboxypeptidase and chymotrypsin.
The enzymes that digest starch (amylase), fat (lipase), and nucleic acids (nuclease) are secreted in their active forms, since they do not attack the pancreas as do the protein-digesting enzymes.
Regulation of pancreatic secretion is the job of hormones and the parasympathetic nervous system. The entry of acidic chyme into the duodenum stimulates the release of secretin, which in turn causes the duct cells to release bicarbonate-rich pancreatic juice. The presence of proteins and fats in the duodenum stimulates the secretion of CCK, which then stimulates the acini to secrete enzyme-rich pancreatic juice and enhances the activity of secretin. Parasympathetic regulation occurs mainly during the cephalic and gastric phases of gastric secretion, when vagal stimulation prompts the secretion of pancreatic juice.
Usually, the pancreas secretes just enough bicarbonate to counterbalance the amount of HCl produced in the stomach. Hydrogen ions enter the blood when bicarbonate is secreted by the pancreas. Thus, the acidic blood draining from the pancreas neutralizes the alkaline blood draining from the stomach, maintaining the pH of the venous blood that flows to the liver.
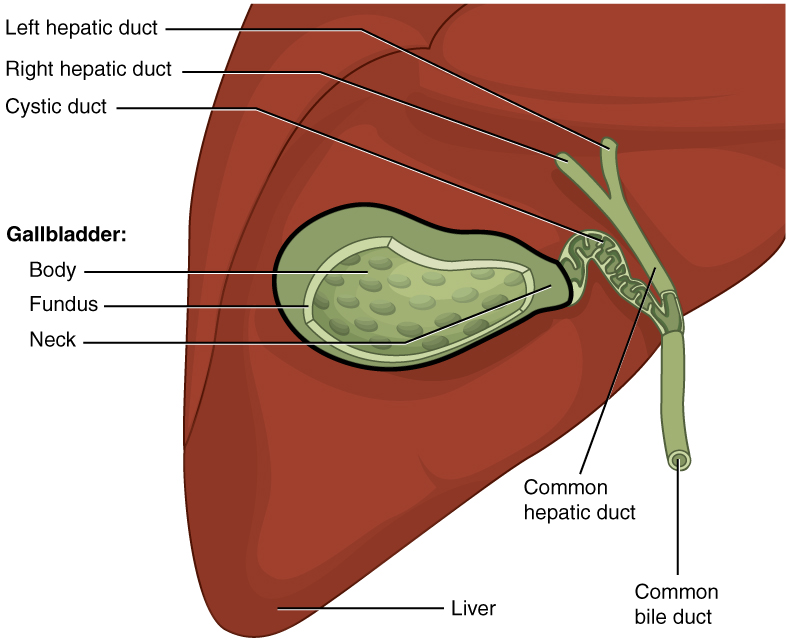
The gallbladder is 8–10 cm (~3–4 in) long and is nested in a shallow area on the posterior aspect of the right lobe of the liver. This muscular sac stores, concentrates, and, when stimulated, propels the bile into the duodenum via the common bile duct. It is divided into three regions. The fundus is the widest portion and tapers medially into the body, which in turn narrows to become the neck. The neck angles slightly superiorly as it approaches the hepatic duct. The cystic duct is 1–2 cm (less than 1 in) long and turns inferiorly as it bridges the neck and hepatic duct.
The simple columnar epithelium of the gallbladder mucosa is organized in rugae, similar to those of the stomach. There is no submucosa in the gallbladder wall. The wall’s middle, muscular coat is made of smooth muscle fibers. When these fibers contract, the gallbladder’s contents are ejected through the cystic duct and into the bile duct ( [link] ). Visceral peritoneum reflected from the liver capsule holds the gallbladder against the liver and forms the outer coat of the gallbladder. The gallbladder's mucosa absorbs water and ions from bile, concentrating it by up to 10-fold.
Chemical digestion in the small intestine cannot occur without the help of the liver and pancreas. The liver produces bile and delivers it to the common hepatic duct. Bile contains bile salts and phospholipids, which emulsify large lipid globules into tiny lipid droplets, a necessary step in lipid digestion and absorption. The gallbladder stores and concentrates bile, releasing it when it is needed by the small intestine.
The pancreas produces the enzyme- and bicarbonate-rich pancreatic juice and delivers it to the small intestine through ducts. Pancreatic juice buffers the acidic gastric juice in chyme, inactivates pepsin from the stomach, and enables the optimal functioning of digestive enzymes in the small intestine.
Watch this video to see the structure of the liver and how this structure supports the functions of the liver, including the processing of nutrients, toxins, and wastes. At rest, about 1500 mL of blood per minute flow through the liver. What percentage of this blood flow comes from the hepatic portal system?
Answers may vary.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?