| << Chapter < Page | Chapter >> Page > |
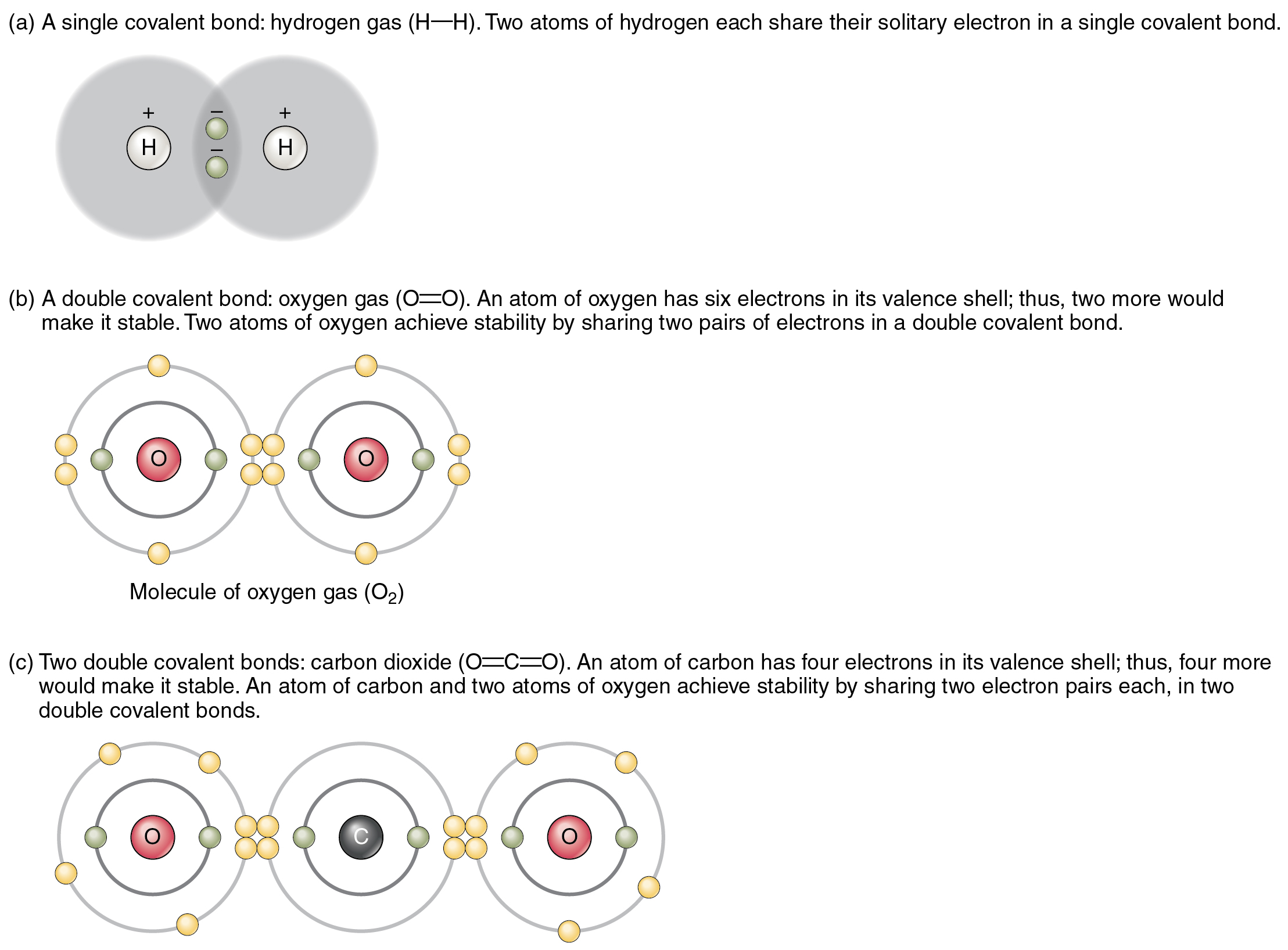
You can see that the covalent bonds shown in [link] are balanced. The sharing of the negative electrons is relatively equal, as is the electrical pull of the positive protons in the nucleus of the atoms involved. This is why covalently bonded molecules that are electrically balanced in this way are described as nonpolar; that is, no region of the molecule is either more positive or more negative than any other.
Groups of legislators with completely opposite views on a particular issue are often described as “polarized” by news writers. In chemistry, a polar molecule is a molecule that contains regions that have opposite electrical charges. Polar molecules occur when atoms share electrons unequally, in polar covalent bonds.
The most familiar example of a polar molecule is water ( [link] ). The molecule has three parts: one atom of oxygen, the nucleus of which contains eight protons, and two hydrogen atoms, whose nuclei each contain only one proton. Because every proton exerts an identical positive charge, a nucleus that contains eight protons exerts a charge eight times greater than a nucleus that contains one proton. This means that the negatively charged electrons present in the water molecule are more strongly attracted to the oxygen nucleus than to the hydrogen nuclei. Each hydrogen atom’s single negative electron therefore migrates toward the oxygen atom, making the oxygen end of their bond slightly more negative than the hydrogen end of their bond.
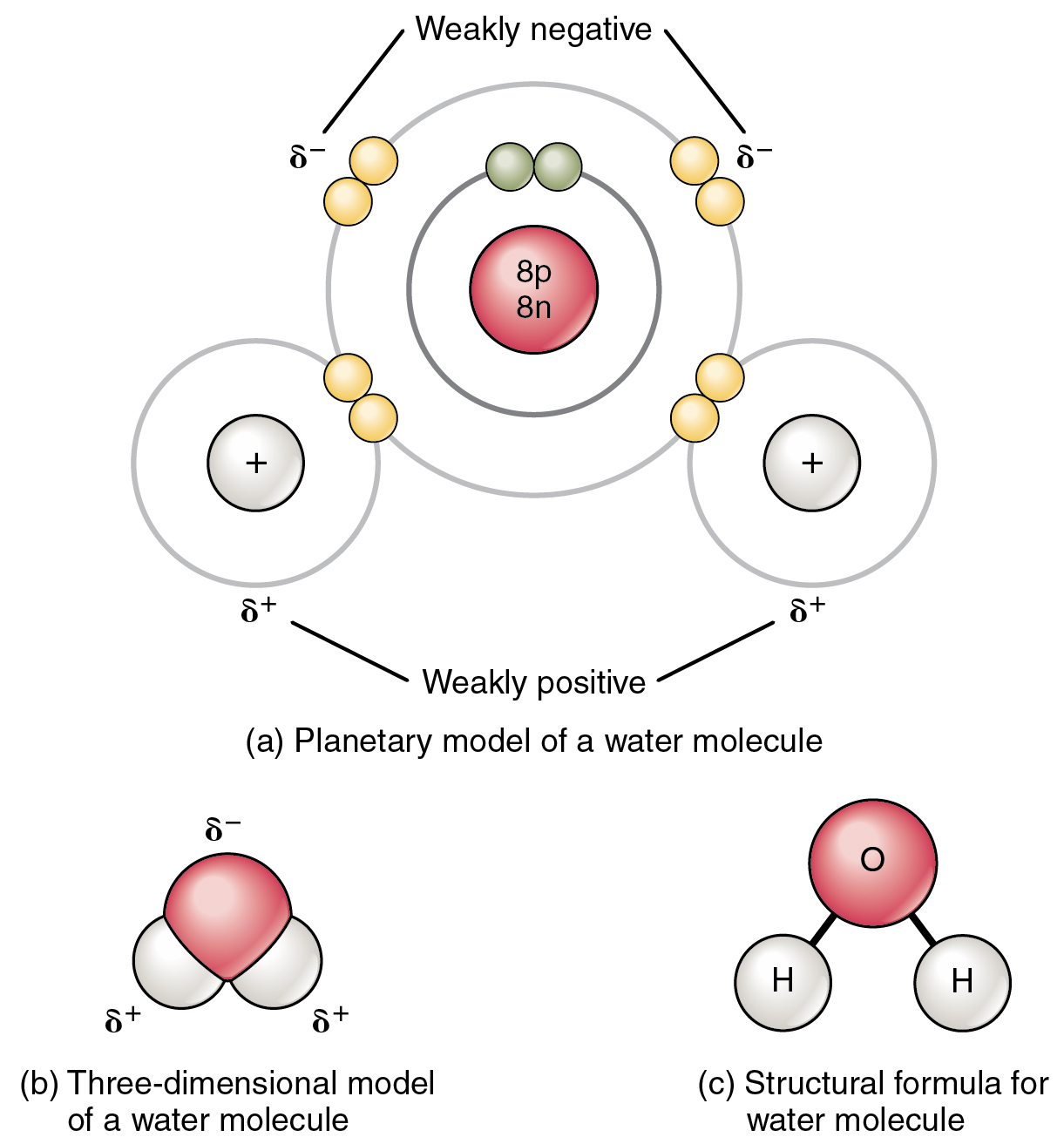
What is true for the bonds is true for the water molecule as a whole; that is, the oxygen region has a slightly negative charge and the regions of the hydrogen atoms have a slightly positive charge. These charges are often referred to as “partial charges” because the strength of the charge is less than one full electron, as would occur in an ionic bond. As shown in [link] , regions of weak polarity are indicated with the Greek letter delta (∂) and a plus (+) or minus (–) sign.
Even though a single water molecule is unimaginably tiny, it has mass, and the opposing electrical charges on the molecule pull that mass in such a way that it creates a shape somewhat like a triangular tent (see [link] b ). This dipole, with the positive charges at one end formed by the hydrogen atoms at the “bottom” of the tent and the negative charge at the opposite end (the oxygen atom at the “top” of the tent) makes the charged regions highly likely to interact with charged regions of other polar molecules. For human physiology, the resulting bond is one of the most important formed by water—the hydrogen bond.
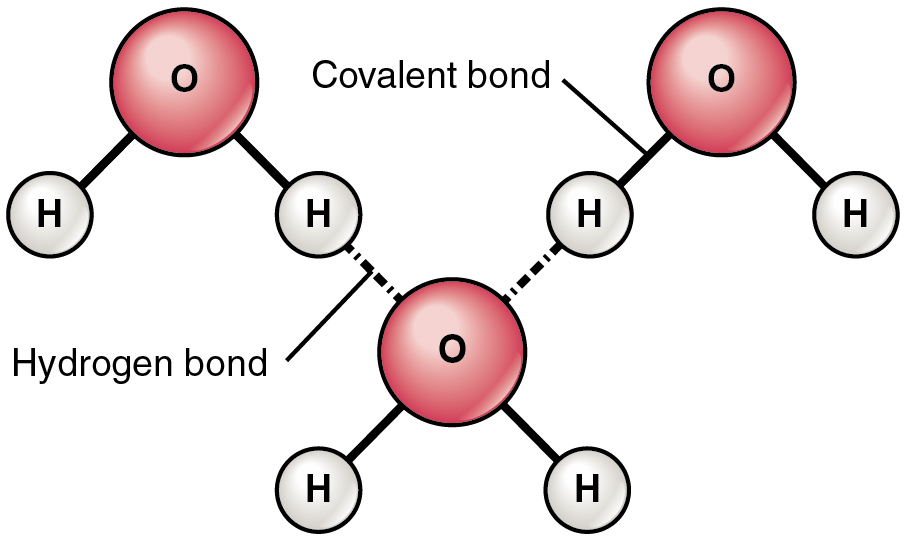
A hydrogen bond is formed when a weakly positive hydrogen atom already bonded to one electronegative atom (for example, the oxygen in the water molecule) is attracted to another electronegative atom from another molecule. In other words, hydrogen bonds always include hydrogen that is already part of a polar molecule.
The most common example of hydrogen bonding in the natural world occurs between molecules of water. It happens before your eyes whenever two raindrops merge into a larger bead, or a creek spills into a river. Hydrogen bonding occurs because the weakly negative oxygen atom in one water molecule is attracted to the weakly positive hydrogen atoms of two other water molecules ( [link] ).
Water molecules also strongly attract other types of charged molecules as well as ions. This explains why “table salt,” for example, actually is a molecule called a “salt” in chemistry, which consists of equal numbers of positively-charged sodium (Na + ) and negatively-charged chloride (Cl – ), dissolves so readily in water, in this case forming dipole-ion bonds between the water and the electrically-charged ions (electrolytes). Water molecules also repel molecules with nonpolar covalent bonds, like fats, lipids, and oils. You can demonstrate this with a simple kitchen experiment: pour a teaspoon of vegetable oil, a compound formed by nonpolar covalent bonds, into a glass of water. Instead of instantly dissolving in the water, the oil forms a distinct bead because the polar water molecules repel the nonpolar oil.
Each moment of life, atoms of oxygen, carbon, hydrogen, and the other elements of the human body are making and breaking chemical bonds. Ions are charged atoms that form when an atom donates or accepts one or more negatively charged electrons. Cations (ions with a positive charge) are attracted to anions (ions with a negative charge). This attraction is called an ionic bond. In covalent bonds, the participating atoms do not lose or gain electrons, but rather share them. Molecules with nonpolar covalent bonds are electrically balanced, and have a linear three-dimensional shape. Molecules with polar covalent bonds have “poles”—regions of weakly positive and negative charge—and have a triangular three-dimensional shape. An atom of oxygen and two atoms of hydrogen form water molecules by means of polar covalent bonds. Hydrogen bonds link hydrogen atoms already participating in polar covalent bonds to anions or electronegative regions of other polar molecules. Hydrogen bonds link water molecules, resulting in the properties of water that are important to living things.
Visit this website to learn about electrical energy and the attraction/repulsion of charges. What happens to the charged electroscope when a conductor is moved between its plastic sheets, and why?
The plastic sheets jump to the nail (the conductor), because the conductor takes on electrons from the electroscope, reducing the repellant force of the two sheets.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?