| << Chapter < Page | Chapter >> Page > |
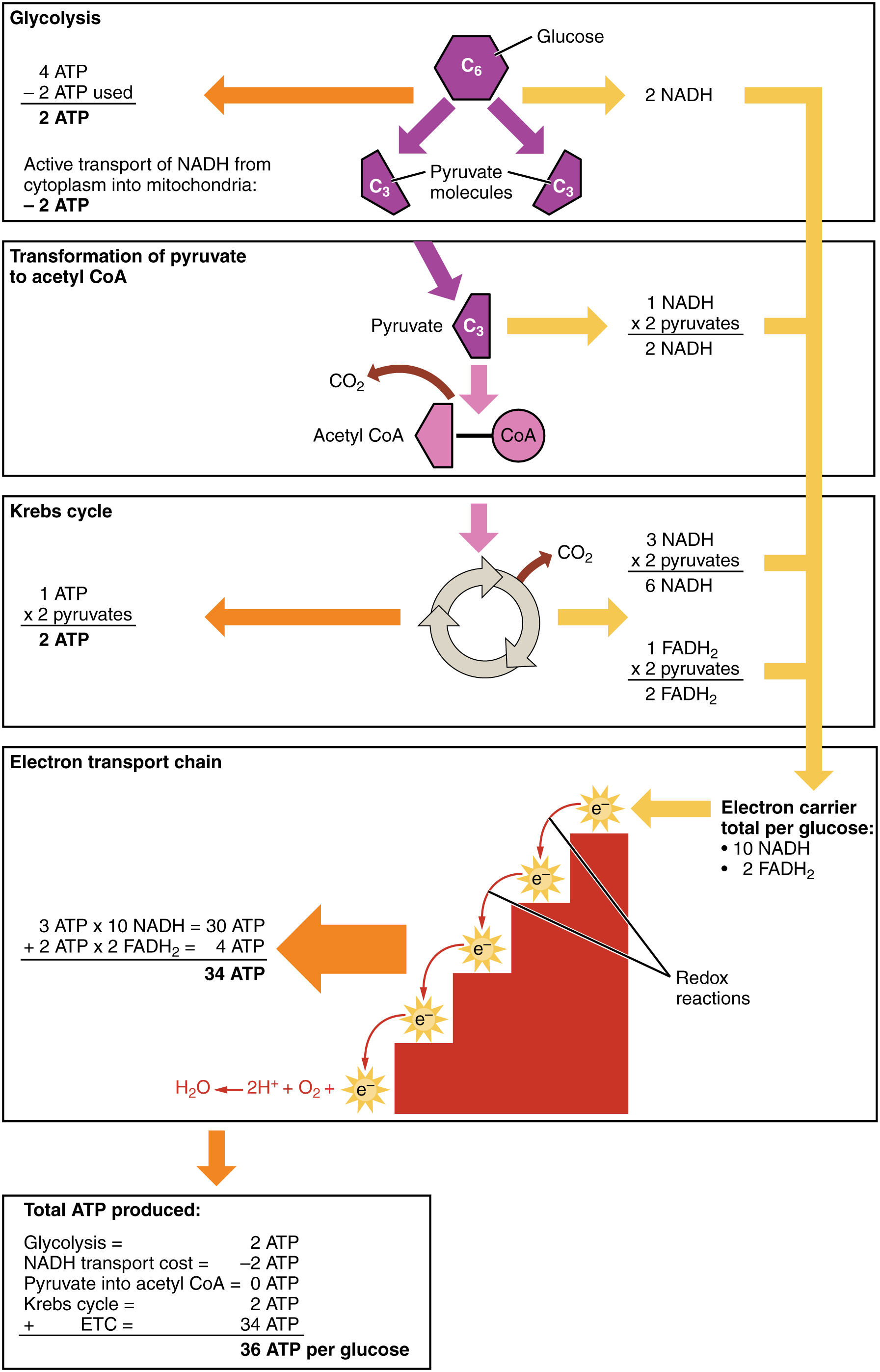
Therefore, for every glucose molecule that enters aerobic respiration, a net total of 36 ATPs are produced ( [link] ).
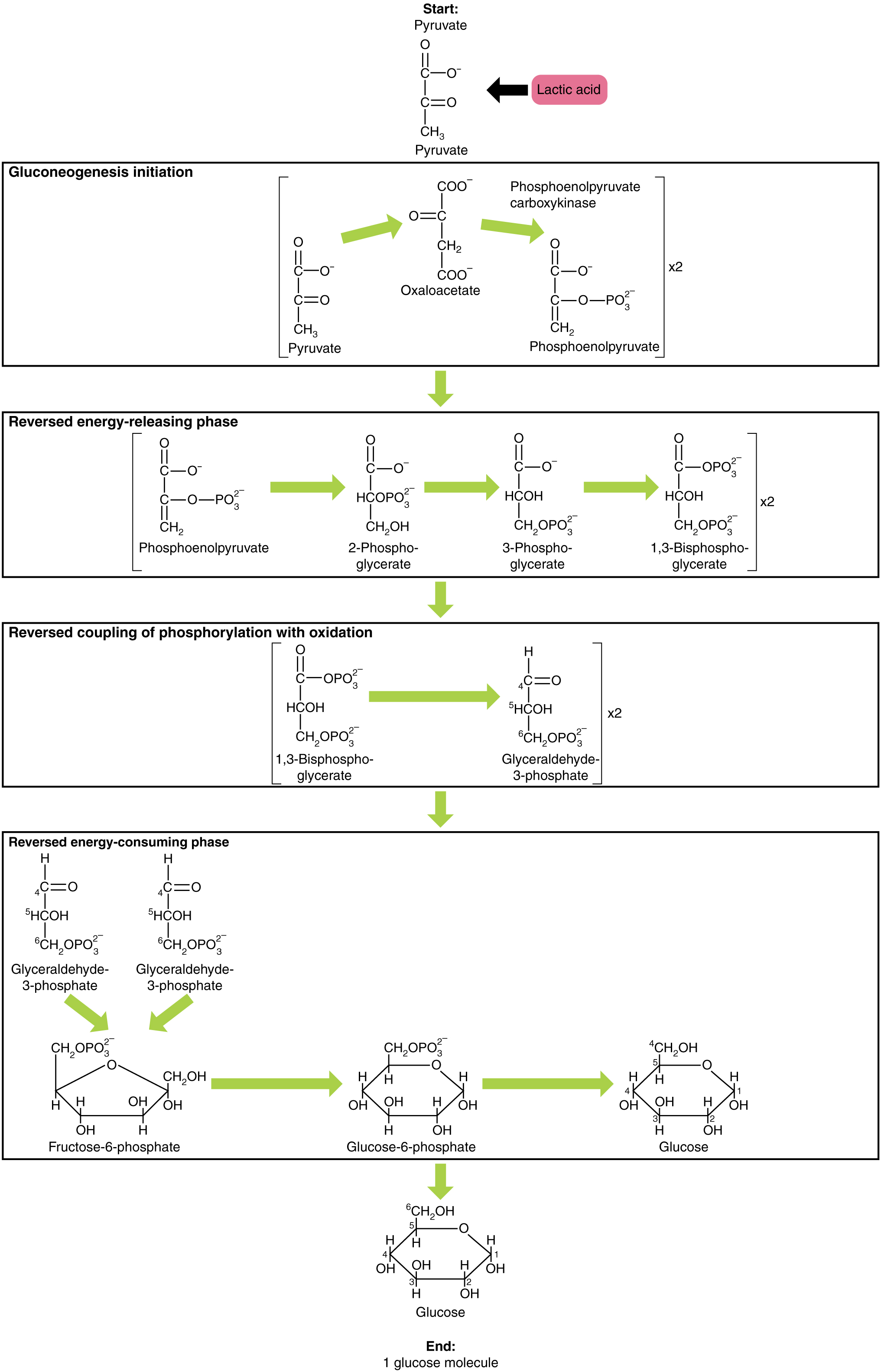
Gluconeogenesis is the synthesis of new glucose molecules from pyruvate, lactate, glycerol, or the amino acids alanine or glutamine. This process takes place primarily in the liver during periods of low glucose, that is, under conditions of fasting, starvation, and low carbohydrate diets. So, the question can be raised as to why the body would create something it has just spent a fair amount of effort to break down? Certain key organs, including the brain, can use only glucose as an energy source; therefore, it is essential that the body maintain a minimum blood glucose concentration. When the blood glucose concentration falls below that certain point, new glucose is synthesized by the liver to raise the blood concentration to normal.
Gluconeogenesis is not simply the reverse of glycolysis. There are some important differences ( [link] ). Pyruvate is a common starting material for gluconeogenesis. First, the pyruvate is converted into oxaloacetate. Oxaloacetate then serves as a substrate for the enzyme phosphoenolpyruvate carboxykinase (PEPCK), which transforms oxaloacetate into phosphoenolpyruvate (PEP). From this step, gluconeogenesis is nearly the reverse of glycolysis. PEP is converted back into 2-phosphoglycerate, which is converted into 3-phosphoglycerate. Then, 3-phosphoglycerate is converted into 1,3 bisphosphoglycerate and then into glyceraldehyde-3-phosphate. Two molecules of glyceraldehyde-3-phosphate then combine to form fructose-1-6-bisphosphate, which is converted into fructose 6-phosphate and then into glucose-6-phosphate. Finally, a series of reactions generates glucose itself. In gluconeogenesis (as compared to glycolysis), the enzyme hexokinase is replaced by glucose-6-phosphatase, and the enzyme phosphofructokinase-1 is replaced by fructose-1,6-bisphosphatase. This helps the cell to regulate glycolysis and gluconeogenesis independently of each other.
As will be discussed as part of lipolysis, fats can be broken down into glycerol, which can be phosphorylated to form dihydroxyacetone phosphate or DHAP. DHAP can either enter the glycolytic pathway or be used by the liver as a substrate for gluconeogenesis.
There are several things that can be done to help prevent general declines in metabolism and to fight back against the cyclic nature of these declines. These include eating breakfast, eating small meals frequently, consuming plenty of lean protein, drinking water to remain hydrated, exercising (including strength training), and getting enough sleep. These measures can help keep energy levels from dropping and curb the urge for increased calorie consumption from excessive snacking. While these strategies are not guaranteed to maintain metabolism, they do help prevent muscle loss and may increase energy levels. Some experts also suggest avoiding sugar, which can lead to excess fat storage. Spicy foods and green tea might also be beneficial. Because stress activates cortisol release, and cortisol slows metabolism, avoiding stress, or at least practicing relaxation techniques, can also help.
Metabolic enzymes catalyze catabolic reactions that break down carbohydrates contained in food. The energy released is used to power the cells and systems that make up your body. Excess or unutilized energy is stored as fat or glycogen for later use. Carbohydrate metabolism begins in the mouth, where the enzyme salivary amylase begins to break down complex sugars into monosaccharides. These can then be transported across the intestinal membrane into the bloodstream and then to body tissues. In the cells, glucose, a six-carbon sugar, is processed through a sequence of reactions into smaller sugars, and the energy stored inside the molecule is released. The first step of carbohydrate catabolism is glycolysis, which produces pyruvate, NADH, and ATP. Under anaerobic conditions, the pyruvate can be converted into lactate to keep glycolysis working. Under aerobic conditions, pyruvate enters the Krebs cycle, also called the citric acid cycle or tricarboxylic acid cycle. In addition to ATP, the Krebs cycle produces high-energy FADH 2 and NADH molecules, which provide electrons to the oxidative phosphorylation process that generates more high-energy ATP molecules. For each molecule of glucose that is processed in glycolysis, a net of 36 ATPs can be created by aerobic respiration.
Under anaerobic conditions, ATP production is limited to those generated by glycolysis. While a total of four ATPs are produced by glycolysis, two are needed to begin glycolysis, so there is a net yield of two ATP molecules.
In conditions of low glucose, such as fasting, starvation, or low carbohydrate diets, glucose can be synthesized from lactate, pyruvate, glycerol, alanine, or glutamate. This process, called gluconeogenesis, is almost the reverse of glycolysis and serves to create glucose molecules for glucose-dependent organs, such as the brain, when glucose levels fall below normal.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?