| << Chapter < Page | Chapter >> Page > |
Predict the electron pair geometry and the molecular structure of each of the following:
(a) IOF 5 (I is the central atom)
(b) POCl 3 (P is the central atom)
(c) Cl 2 SeO (Se is the central atom)
(d) ClSO + (S is the central atom)
(e) F 2 SO (S is the central atom)
(f)
(g)
Which of the following molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
(a) ClF 5
(b)
(c)
(d) PCl 3
(e) SeF 4
(f)
(g) XeF 2
All of these molecules and ions contain polar bonds. Only ClF 5 , PCl 3 , SeF 4 , and have dipole moments.
Which of these molecules and ions contain polar bonds? Which of these molecules and ions have dipole moments?
(a) H 3 O +
(b)
(c)
(d)
(e) ICl 3
(f) XeF 4
(g) SF 2
Which of the following molecules have dipole moments?
(a) CS 2
(b) SeS 2
(c) CCl 2 F 2
(d) PCl 3 (P is the central atom)
(e) ClNO (N is the central atom)
SeS 2 , CCl 2 F 2 , PCl 3 , and ClNO all have dipole moments.
Identify the molecules with a dipole moment:
(a) SF 4
(b) CF 4
(c) Cl 2 CCBr 2
(d) CH 3 Cl
(e) H 2 CO
The molecule XF 3 has a dipole moment. Is X boron or phosphorus?
P
The molecule XCl 2 has a dipole moment. Is X beryllium or sulfur?
There are three possible structures for PCl 2 F 3 with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.
Describe the molecular structure around the indicated atom or atoms:
(a) the sulfur atom in sulfuric acid, H 2 SO 4 [(HO) 2 SO 2 ]
(b) the chlorine atom in chloric acid, HClO 3 [HOClO 2 ]
(c) the oxygen atom in hydrogen peroxide, HOOH
(d) the nitrogen atom in nitric acid, HNO 3 [HONO 2 ]
(e) the oxygen atom in the OH group in nitric acid, HNO 3 [HONO 2 ]
(f) the central oxygen atom in the ozone molecule, O 3
(g) each of the carbon atoms in propyne, CH 3 CCH
(h) the carbon atom in Freon, CCl 2 F 2
(i) each of the carbon atoms in allene, H 2 CCCH 2
(a) tetrahedral; (b) trigonal pyramidal; (c) bent (109°); (d) trigonal planar; (e) bent (109°); (f) bent (109°); (g) C H 3 CCH tetrahedral, CH 3 CC H linear; (h) tetrahedral; (i) H 2 C C CH 2 linear; H 2 C C C H 2 trigonal planar
Draw the Lewis structures and predict the shape of each compound or ion:
(a) CO 2
(b)
(c) SO 3
(d)
A molecule with the formula AB 2 , in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.

A molecule with the formula AB 3 , in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion that has each shape.
Draw the Lewis electron dot structures for these molecules, including resonance structures where appropriate:
(a)
(b) CS 2
(c) CS
(d) predict the molecular shapes for and CS 2 and explain how you arrived at your predictions
(a)
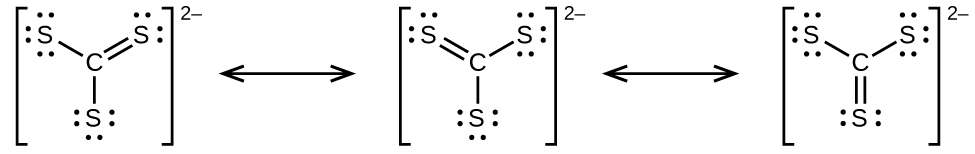 ;
;
(b)
 ;
;
(c)
![]() ;
;
(d)
includes three regions of electron density (all are bonds with no lone pairs); the shape is trigonal planar; CS
2 has only two regions of electron density (all bonds with no lone pairs); the shape is linear
What is the molecular structure of the stable form of FNO 2 ? (N is the central atom.)
A compound with a molar mass of about 42 g/mol contains 85.7% carbon and 14.3% hydrogen. What is its molecular structure?
The Lewis structure is made from three units, but the atoms must be rearranged:
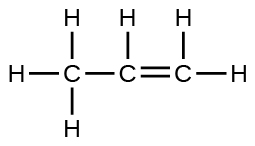
Use the simulation to perform the following exercises for a two-atom molecule:
(a) Adjust the electronegativity value so the bond dipole is pointing toward B. Then determine what the electronegativity values must be to switch the dipole so that it points toward A.
(b) With a partial positive charge on A, turn on the electric field and describe what happens.
(c) With a small partial negative charge on A, turn on the electric field and describe what happens.
(d) Reset all, and then with a large partial negative charge on A, turn on the electric field and describe what happens.
Use the simulation to perform the following exercises for a real molecule. You may need to rotate the molecules in three dimensions to see certain dipoles.
(a) Sketch the bond dipoles and molecular dipole (if any) for O 3. Explain your observations.
(b) Look at the bond dipoles for NH 3 . Use these dipoles to predict whether N or H is more electronegative.
(c) Predict whether there should be a molecular dipole for NH 3 and, if so, in which direction it will point. Check the molecular dipole box to test your hypothesis.
The molecular dipole points away from the hydrogen atoms.
Use the Molecule Shape simulator to build a molecule. Starting with the central atom, click on the double bond to add one double bond. Then add one single bond and one lone pair. Rotate the molecule to observe the complete geometry. Name the electron group geometry and molecular structure and predict the bond angle. Then click the check boxes at the bottom and right of the simulator to check your answers.
Use the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select H 2 O. Switch between the “real” and “model” modes. Explain the difference observed.
The structures are very similar. In the model mode, each electron group occupies the same amount of space, so the bond angle is shown as 109.5°. In the “real” mode, the lone pairs are larger, causing the hydrogens to be compressed. This leads to the smaller angle of 104.5°.
Use the Molecule Shape simulator to explore real molecules. On the Real Molecules tab, select “model” mode and S 2 O. What is the model bond angle? Explain whether the “real” bond angle should be larger or smaller than the ideal model angle.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?